Abstract
Aims/hypothesis
Type 1 diabetes is a common autoimmune disease that has genetic and environmental determinants. Variations within the IL2 and IL2RA (also known as CD25) gene regions are associated with disease risk, and variation in expression or function of these proteins is likely to be causal. We aimed to investigate if circulating concentrations of the soluble form of CD25, sCD25, an established marker of immune activation and inflammation, were increased in individuals with type 1 diabetes and if this was associated with the concentration of C-peptide, a measure of insulin production that reflects the degree of autoimmune destruction of the insulin-producing beta cells.
Methods
We used immunoassays to measure sCD25 and C-peptide in peripheral blood plasma from patient and control samples.
Results
We identified that sCD25 was increased in patients with type 1 diabetes compared with controls and replicated this result in an independent set of 86 adult patient and 80 age-matched control samples (p = 1.17 × 10−3). In 230 patients under 20 years of age, with median duration-of-disease of 6.1 years, concentrations of sCD25 were negatively associated with C-peptide concentrations (p = 4.8 × 10−3).
Conclusions/interpretation
The 25% increase in sCD25 in patients, alongside the inverse association between sCD25 and C-peptide, probably reflect the adverse effects of an on-going, actively autoimmune and inflammatory immune system on beta cell function in patients.
Similar content being viewed by others
Introduction
The IL-2/IL-2 receptor α (IL-2RA) signalling pathway is essential for the regulation of immune responses. Targeted disruption of IL-2 and IL-2RA in mice causes systemic autoimmune disease [1, 2], as do rare IL2RA mutations in humans [3, 4]. IL-2RA (CD25) is expressed on many haematopoietic cells, including subsets of T and B cells, most notably regulatory T cells (Tregs), dendritic cells and monocytes, and also non-haematopoietic cells such as endothelial cells, and is upregulated on activation of these cells [5, 6]. The IL-2RA subunit is essential for high-affinity binding of IL-2, and unlike the IL-2RB subunit and the common cytokine receptor γ chain, which bind to other cytokines, the α subunit is unique to IL-2 [6]. IL-2 is largely produced by activated T cells and is required for the generation of functional Tregs [7] and peripheral Treg fitness and maintenance [8, 9].
Upon activation, immune cells proliferate and CD25 is cleaved from the surface by proteases [10–13], including matrix metalloproteinase-2 (MMP-2) and MMP-9 [14–16]. Inhibition of these proteases decreases CD25 cleavage, thus increasing the stability of surface CD25 in vitro [17]. The concentration of sCD25 is age dependent in healthy children, who have high circulating sCD25 concentrations that fall to normal adult concentrations (∼2,000 pg/ml) by age 16–18 years [18]. Elevated sCD25 concentrations in adults are associated with activation of lymphocytes during infection and inflammation, and with autoimmune disease [19–22]. Therefore, sCD25 has been used as a biomarker to help characterise disease progression, prognosis and treatment [23–25]. A previous study of 35 patients with newly diagnosed type 1 diabetes and age-matched controls showed that patients had higher sCD25 concentrations [21]. However, others have reported conflicting results [26, 27].
sCD25 binds to IL-2 in vitro, but with a low affinity (K d = 0.03 mol/l) compared with IL-2Rαβγ complex binding of IL-2 (K d = 10−11 mol/l) [28, 29]. Experiments have shown that, at high concentrations, sCD25 may block IL-2 signalling in vitro [17, 25, 30]. However, at lower concentrations, sCD25 has been shown to potentiate IL-2 signalling [31], as observed with the ligands of other soluble cytokine receptors [32, 33]. Owing to the essential role of IL-2 and the IL-2/IL-2RA pathway for immune homeostasis, the mechanism for cleavage of CD25 from the cell surface and the concentration of sCD25 in the periphery may have an immunoregulatory role and/or indicate immune activation and inflammation.
Here we have measured the concentration of circulating sCD25 in plasma samples from adult (>18 years) patients with type 1 diabetes and healthy adult controls to determine if sCD25 concentrations are associated with disease. Impaired beta cell insulin secretion in patients can be assessed using C-peptide measurements [34]. C-peptide is co-secreted with insulin by the pancreas, as a by-product of the enzymatic cleavage of proinsulin to insulin, and, in patients diagnosed with type 1 diabetes, C-peptide levels decline rapidly because of the autoimmune destruction or inactivation of beta cells. Using C-peptide measurements, we aimed to assess whether there was an association between sCD25 and residual beta cell function in young people with childhood-onset type 1 diabetes and variable diabetes duration.
Methods
Samples
For the initial case–control analysis, 200 plasma samples were used from adult patients with type 1 diabetes collected as part of the JDRF/Wellcome Trust GRID cohort (www-gene.cimr.cam.ac.uk/todd/, accessed 1 January 2012). Patients, who were of self-reported white ethnicity, were diagnosed under 17 years of age, and plasma samples were acquired over the age of 18 years. Plasma samples for the 1,600 adult controls were collected as part of the UK Blood Services Common Control Collection (UKBS-CC) [35]. GRID and UKBS-CC samples were collected in acid citrate dextrose solution anticoagulant (Table 1).
For the replication study of the case–control analysis, plasma samples from 86 patients and 80 age-matched controls were collected through the Cambridge BioResource (CBR) [5]. All samples for the replication study were collected with the same protocol using EDTA anticoagulant (Table 1).
Non-fasting serum samples for measuring both C-peptide and sCD25 levels were available from 230 young people followed in the Nephropathy Family Study (NFS). The NFS is a prospective study that has, since 2000, recruited more than 1,000 adolescents (10–18 years) with type 1 diabetes and has followed them longitudinally [36]. For the present analysis, 230 patients (age 10–20 years) with variable type 1 diabetes duration, had an available stored serum sample (Table 1). The concentrations of both sCD25 and C-peptide were measured in these samples.
Ethics approval was obtained from the ethics committee, with written consent from participants or parents with assent from the children. All data and samples are treated as confidential. Samples and data are identifiable by a unique barcode only, and volunteers are free to withdraw from these projects at any time. All plasma and serum samples were stored at −80°C.
sCD25 concentrations
Plasma or serum samples were assayed for sCD25 concentrations using BD OptEIA Human ELISA Kit (BD Biosciences, Franklin Lakes, NJ, USA). The recommended protocol was modified to incorporate mouse IgG, at 10 μg/ml, within the sample diluent.
Europium-labelled streptavidin combined with time-resolved fluorescence spectroscopy was used as the assay readout using DELFIA reagents (Perkin Elmer, Waltham, MA, USA). Each sample was diluted 1:20 in duplicate, and each 100 μl dilution was assayed in the same 96-well plate. Each 96-well plate contained a recombinant sCD25 protein standard curve with a detection range of 31–500 pg/ml. Within the 1,600 UKBS control samples, the mean CV between duplicates was 5.00%.
To assess the reproducibility of the immunoassay, we measured sCD25 concentrations in 40 patient and 40 control plasma samples, in two independent experiments. The two sCD25 concentrations correlated (r = 0.86), indicating good reproducibility. In adults, the concentration of sCD25 has been shown to be stable over 12 months [25]. To substantiate this, we measured sCD25 concentrations in 13 adults, with two plasma samples acquired over 6 months apart (mean 236 days). We observed that sCD25 concentrations were stable over this time period (r = 0.86). The background level of reactivity, possibly caused by heterophile antibodies, was measured using a mismatched IL-7R detection antibody (human CD127 [IL-7R] biotinylated antibody; eBiosciences, San Diego, CA, USA ) in combination with the standard sCD25 primary antibody. No correlation was observed with sCD25 concentration, and the background concentration was measured using the IL-7R detection antibody in the 40 patient and 40 control plasma samples tested (r = 0.03 and r = 0.001, respectively).
C-peptide concentration
C-peptide concentrations were measured using a 1235 AutoDELFIA automatic immunoassay kit from Perkin Elmer . The lower limit of detection was 6.6 pmol/l, and samples with this value were included in analyses and not censored unless described.
Samples were assayed in singleton on a system using a two-step time-resolved fluorimetric assay. All reagents, standards and consumables were those recommended and supplied by the manufacturer. Cross-reactivity with intact proinsulin and 32-33 split proinsulin is ∼60% at 400 pmol/l. Cross-reactivity with intact insulin is <0.1% at 6,000 pmol/l. Between-batch imprecision was 4.0% at 190 pmol/l, 3.8% at 1,125 pmol/l, 1.9% at 277 pmol/l, and 2.9% at 3,898 pmol/l (in-house data).
Statistical analysis
After graphical examination, sCD25 and C-peptide concentrations were log10 transformed to generate a more symmetrical distribution for statistical analysis. To evaluate their relationships with covariates, log10 sCD25 or log10 C-peptide was used as the dependent variable in multiple linear regressions, with the appropriate covariates as independent variables and Wald tests.
For the initial case–control analysis, sCD25 was measured in GRID type 1 diabetic patients and UKBS control samples, which were randomly split over two ELISA batches. Log10 sCD25 concentrations did not differ by batch (p = 0.98; type 1 diabetes status was not taken into account because we randomised cases and controls across the two batches). It has been previously shown that sCD25 concentrations are stable in adults [18]. Therefore, we tested this in the 1,600 UKBS control samples and confirmed that sCD25 concentrations in individuals over 18 years of age were not associated with age (p = 0.66). As age and batch were not associated with sCD25 concentrations in this dataset, neither was required as covariates in the analysis.
We used regression analysis to compare log-transformed sCD25 concentrations from patient and control samples for the initial analysis of 200 patient and 1,600 control samples, and for the replication analysis of 86 patient and 80 control samples. As the replication samples were age-matched, we also repeated the analysis on a subset of 77 pairs, where both samples had sCD25 and C-peptide concentrations, using a paired t test to account for the matching.
For the comparative analysis of sCD25 and C-peptide, up to two serum samples from 230 NFS patients were measured for sCD25 and C-peptide concentrations. Using multiple linear regression analysis, we identified covariates that explained variance in log10 sCD25 and log10 C-peptide concentrations (age-at-diagnosis and duration-of-disease, respectively; electronic supplementary material [ESM] Table 1, ESM Figs 1 and 2). The linear model we fit assumes a constant rate of change in log10 C-peptide concentrations with time since diagnosis. This can only be an approximation to the underlying biological reality, as there must come a time when C-peptide stops decreasing. However, when we attempted to include additional polynomial terms to allow for this, the resulting model predicted that C-peptide levels would start increasing some 7 years after diagnosis. As this is biologically unsound, we chose to use the linear model to adjust log10 sCD25 for age-at-diagnosis and log10 C-peptide concentrations for duration-of-disease. We then tested whether the log10 C-peptide residual (‘adjusted log10 C-peptide’) was a significant predictor of the log10 sCD25 residual (‘adjusted log10 sCD25’) in the 230 samples.
Results
Elevated sCD25 concentrations in type 1 diabetes
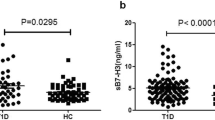
We tested for association of sCD25 concentration with type 1 diabetes status using samples from adult patients with type 1 diabetes and controls. Type 1 diabetes was associated with log10 sCD25 concentration (p = 3.12 × 10−16), with concentrations higher in the 200 patient samples than in the 1,600 control samples (Fig. 1a).
Log10 sCD25 concentrations were higher in adult type 1 diabetic patients than in adult control samples. (a) 200 type 1 diabetic patients and 1,600 control samples (p = 3.12 × 10−16) and (b) 86 type 1 diabetic patients and 80 control samples (p = 1.17 × 10−3). Grey bars, type 1 diabetic patients; white bars, controls
Differences in sample collection and/or processing between the patient and control plasma samples could confound this initial observation. Therefore, in order to replicate these findings, we measured sCD25 concentrations in an independent set of 86 type 1 diabetic patients and 80 age-matched adult controls collected and processed using the same protocol. In this replication dataset, log10 sCD25 concentration was associated with disease status, with higher concentrations observed in the type 1 diabetic patient samples (mean 4,211 pg/ml) compared with controls (mean 3,356 pg/ml; p = 1.17 × 10−3; Fig. 1b). This difference was maintained in the subset of 77 matched pairs using a paired t test (mean difference 0.091 [95% CI 0.041, 0.140], t = 3.67, df = 76, p = 4.52 × 10−4).
Increased sCD25 concentrations are associated with decreasing C-peptide concentrations in patients with type 1 diabetes
To determine if sCD25 concentrations were associated with C-peptide concentrations, we analysed measurements from 230 NFS patient samples. The median age was 14.7 years, median duration-of-disease 6.07 years, and 59% of these NFS patients were male. Log10 sCD25 concentrations were associated with log10 C-peptide concentrations and explained 3.39% of the variance observed (p = 4.8 × 10−3, regression coefficient −0.051 [95% CI −0.087, −0.02]; Fig. 2). We repeated this analysis, dropping samples at the lower limit of detection for C-peptide concentration (ESM Fig. 2). Within the remaining 181 samples, the association between log10 sCD25 concentrations and log10 C-peptide concentrations was still observed, with a similar regression coefficient (regression coefficient −0.068 [95% CI −0.117, −0.019], p = 6.70 × 10−3).
Discussion
Here we have measured sCD25 concentrations in type 1 diabetic patient and control samples to test if sCD25 concentrations were associated with disease. Circulating sCD25 is used as a biomarker for immune activation [23–25], and previous experiments have also suggested a possible regulatory role for the sCD25 molecule in IL-2 signalling [17, 25, 31].
We know that genetic variation in the IL2RA region is associated with susceptibility to type 1 diabetes [37–39], multiple sclerosis [40, 41], Graves’ disease [42], rheumatoid arthritis [43], Crohn’s disease [44], systemic lupus erythematosus [45] and juvenile idiopathic arthritis [46]. Multiple independent association signals within the IL2RA region confer risk to type 1 diabetes and are associated with sCD25 concentrations [38, 47, 48]. However, we have observed allelic heterogeneity between the IL2RA variants associated with type 1 diabetes and sCD25 concentration [42], and, until much more detailed genetic mapping in larger sample sets is carried out, it will remain unclear if there are causal variants at IL2RA shared between sCD25 concentration and risk of type 1 diabetes and other immune diseases.
As sCD25 is associated with age in under 18 year olds, we used adults for our case–control experiments. We identified and replicated an association between sCD25 concentration and disease status, with concentrations 25% higher in adult patients with type 1 diabetes compared with adult control samples (Fig. 1b). This result indicates that sCD25 can be used as a marker for immune activation in patients with, or those at high risk of, type 1 diabetes.
We hypothesised that patients with higher sCD25 concentrations may have more aggressive on-going immune destruction of the pancreatic beta cells and consequently less C-peptide. In samples from 230 patients with type 1 diabetes under 20 years old, we identified that C-peptide concentrations were inversely associated with sCD25 concentrations even in patients with long-duration type 1 diabetes (Fig. 2). These results are based on a small group of young people with type 1 diabetes, and there is a potential limitation related to the measurement of C-peptide, which was performed on non-fasting samples and therefore need to be confirmed in larger studies, including a better assessment of beta cell function. Nevertheless, our findings suggest that sCD25 concentrations could be used as markers for C-peptide loss, and sCD25 could be an informative marker to monitor in immunotherapeutic trials to intervene in the progression of the disease after diagnosis. Our results also add to the large body of literature that indicates a role for immune activation and proinflammatory cytokines, such as TNF-α, IL-1 and IL-6, in the promotion of beta cell death in type 1 diabetes [49]. Rather than sCD25 itself being causal, we think it likely that a raised sCD25 concentration in some patients is a downstream consequence of an active autoimmune inflammatory process.
Abbreviations
- CBR:
-
Cambridge BioResource
- IL-2RA:
-
IL-2 receptor α
- MMP:
-
Matrix metalloproteinase
- NFS:
-
Nephropathy Family Study
- sCD25:
-
Soluble CD25
- Treg:
-
Regulatory T cell
- UKBS-CC:
-
UK Blood Services Common Control Collection
References
Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75:253–261
Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW (1995) Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3:521–530
Sharfe N, Dadi HK, Shahar M, Roifman CM (1997) Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A 94:3168–3171
Goudy K, Aydin D, Barzaghi F et al (2013) Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol 146:248–261
Dendrou CA, Plagnol V, Fung E et al (2009) Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet 41:1011–1015
Krieg C, Letourneau S, Pantaleo G, Boyman O (2010) Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 107:11906–11911
Malek TR, Castro I (2010) Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33:153–165
Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6:1142–1151
Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA (2007) IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 178:280–290
Rubin LA, Kurman CC, Fritz ME et al (1985) Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135:3172–3177
Rubin LA, Galli F, Greene WC, Nelson DL, Jay G (1990) The molecular basis for the generation of the human soluble interleukin 2 receptor. Cytokine 2:330–336
Kniep EM, Strelow I, Lohmann-Matthes ML (1992) The monocyte interleukin-2 receptor light chain: production of cell-associated and soluble interleukin-2 receptor by monocytes. Immunology 75:299–304
Holter W, Goldman CK, Casabo L, Nelson DL, Greene WC, Waldmann TA (1987) Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol 138:2917–2922
Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH (2001) A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 61:237–242
Schulz O, Sewell HF, Shakib F (1998) Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med 187:271–275
Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz HJ, Ansorge S (1999) Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytokine Res 19:1277–1287
Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA (2009) Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PLoS One 4:e7980
Bien E, Rapala M, Krawczyk M, Balcerska A (2010) The serum levels of soluble interleukin-2 receptor alpha and lactate dehydrogenase but not of B2-microglobulin correlate with selected clinico-pathological prognostic factors and response to therapy in childhood soft tissue sarcomas. J Cancer Res Clin Oncol 136:293–305
Novikov VV, Egorova NI, Kurnikov GY, Evsegneeva IV, Baryshnikov AY, Karaulov AV (2007) Serum levels of soluble HLA and IL-2R molecules in patients with urogenital chlamydia infection. Adv Exp Med Biol 601:285–289
Makis AC, Galanakis E, Hatzimichael EC, Papadopoulou ZL, Siamopoulou A, Bourantas KL (2005) Serum levels of soluble interleukin-2 receptor alpha (sIL-2Ralpha) as a predictor of outcome in brucellosis. J Infect 51:206–210
Giordano C, Galluzzo A, Marco A et al (1988) Increased soluble interleukin-2 receptor levels in the sera of type 1 diabetic patients. Diabetes Res 8:135–138
Greenberg SJ, Marcon L, Hurwitz BJ, Waldmann TA, Nelson DL (1988) Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N Engl J Med 319:1019–1020
Davas EM, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis PC (1999) Serum IL-6, TNFalpha, p55 srTNFalpha, p75srTNFalpha, srIL-2alpha levels and disease activity in systemic lupus erythematosus. Clin Rheumatol 18:17–22
ter Borg EJ, Horst G, Limburg PC, Kallenberg CG (1990) Changes in plasma levels of interleukin-2 receptor in relation to disease exacerbations and levels of anti-dsDNA and complement in systemic lupus erythematosus. Clin Exp Immunol 82:21–26
Maier LM, Anderson DE, Severson CA et al (2009) Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol 182:1541–1547
Wagner R, Bonifacio E, Bingley PJ, Genovese S, Reinwein D, Bottazzo GF (1994) Low interleukin-2 receptor levels in serum of patients with insulin-dependent diabetes. Clin Investig 72:494–498
Vialettes B, Schmitt N, Hirn M et al (1991) The soluble receptor of interleukin 2 is not a serum marker of the autoimmune activity in type I diabetes mellitus. Autoimmunity 11:53–59
Robb RJ, Kutny RM (1987) Structure–function relationships for the IL 2-receptor system. IV. Analysis of the sequence and ligand-binding properties of soluble Tac protein. J Immunol 139:855–862
Matsuoka M, Takeshita T, Ishii N, Nakamura M, Ohkubo T, Sugamura K (1993) Kinetic study of interleukin-2 binding on the reconstituted interleukin-2 receptor complexes including the human gamma chain. Eur J Immunol 23:2472–2476
Russell SE, Moore AC, Fallon PG, Walsh PT (2012) Soluble IL-2Ralpha (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLoS One 7:e47748
Yang ZZ, Grote DM, Ziesmer SC et al (2011) Soluble IL-2R{alpha} facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B cell non-Hodgkin lymphoma. Blood 118:2809–2820
Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM (2001) The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 15:43–58
Rubinstein MP, Kovar M, Purton JF et al (2006) Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U S A 103:9166–9171
Sherry NA, Tsai EB, Herold KC (2005) Natural history of beta-cell function in type 1 diabetes. Diabetes 54(Suppl 2):S32–S39
Consortium WTCC (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447:661–678
Marcovecchio ML, Dalton RN, Schwarze CP et al (2009) Ambulatory blood pressure measurements are related to albumin excretion and are predictive for risk of microalbuminuria in young people with type 1 diabetes. Diabetologia 52:1173–1181
Vella A, Cooper JD, Lowe CE et al (2005) Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet 76:773–779
Lowe CE, Cooper JD, Brusko T et al (2007) Large-scale genetic fine mapping and genotype–phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 39:1074–1082
Qu HQ, Montpetit A, Ge B, Hudson TJ, Polychronakos C (2007) Toward further mapping of the association between the IL2RA locus and type 1 diabetes. Diabetes 56:1174–1176
Hafler DA, Compston A, Sawcer S et al (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357:851–862
Matesanz F, Caro-Maldonado A, Fedetz M et al (2007) IL2RA/CD25 polymorphisms contribute to multiple sclerosis susceptibility. J Neurol 254:682–684
Brand OJ, Lowe CE, Heward JM et al (2007) Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 66:508–512
Barton A, Thomson W, Ke X et al (2008) Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat Genet 40:1156–1159
Franke A, McGovern DPB, Barrett JC et al (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42:1118–1125
Harley JB, Alarcon-Riquelme ME, Criswell LA et al (2008) Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 40:204–210
Hinks A, Cobb J, Marion MC et al (2013) Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet 45:664–669
Maier LM, Lowe CE, Cooper J et al (2009) IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet 5:e1000322
Chistiakov DA, Chistiakova EI, Voronova NV, Turakulov RI, Savost'anov KV (2011) A variant of the Il2ra / Cd25 gene predisposing to Graves' disease is associated with increased levels of soluble interleukin-2 receptor. Scand J Immunol 74:496–501
Pham MN, Kolb H, Battelino T et al (2013) Fasting and meal-stimulated residual beta cell function is positively associated with serum concentrations of proinflammatory cytokines and negatively associated with anti-inflammatory and regulatory cytokines in patients with longer term type 1 diabetes. Diabetologia 56:1356–1363
Acknowledgements
We acknowledge use of samples from the GRID collection and thank the British Society for Paediatric Endocrinology and Diabetes. We acknowledge use of samples from the UK Blood Services collection of Common Controls (UKBS collection). The collection was established as part of the Wellcome Trust Case–Control Consortium. We acknowledge the study field workers, paediatricians, physicians and diabetes nurse specialists involved in NFS and the National Institute for Health Research (NIHR) Cambridge Comprehensive Biomedical Research Centre. We thank members of the Cambridge BioResource Management Committee and Scientific Advisory Board. We thank K. Beer, P. Tagart and M. Wiesner of the Cambridge BioResource for blood sample collection, and M. Woodburn and T. Attwood of the JDRF/Wellcome Trust Diabetes and Inflammation Laboratory for their contribution to sample management. We also thank G. Coleman, S. Duley, S. Hawkins, M. Maisuria, T. Mistry and N. Taylor, of the JDRF/Wellcome Trust Diabetes and Inflammation Laboratory, for preparation of samples. We gratefully acknowledge all study participants.
Funding
This work was supported by the JDRF, the Wellcome Trust (061858, 076113 and 091157), the National Institute for Health Research Cambridge Biomedical Research Centre and the JDRF UK Centre for Diabetes – Genes, Autoimmunity and Prevention Grant (4-2007-1003). The research leading to these results has received funding from the European Union’s 7th Framework Programme (FP7/2007-2013) under grant agreement no. 241447 (NAIMIT). The Cambridge Institute for Medical Research (CIMR) is in receipt of a Wellcome Trust Strategic Award (100140). The NFS is funded by the JDRF, the Wellcome Trust and Diabetes UK. CW is funded by the Wellcome Trust (089989).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
KD, MLM, DBD and JAT conceived the experimental design and interpreted the data and wrote the manuscript. KD, MLM, SN, HES, JJ and PC acquired data. KD, JDC, RCF, JMMH, NMW and CW analysed data. All authors reviewed/edited the manuscript and gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
Log10 sCD25 concentration was associated with age-at-diagnosis in the 230 NFS type diabetes patient samples. Variance explained = 5.1%, regression coefficient = -0.0098 (95% CI = -0.015, - 0.005), p = 1.96 x 10-4(PDF 156 kb)
ESM Fig. 2
Log10 C-peptide concentration was associated with duration-of-disease in the 230 NFS samples. Variance explained = 13.6%, regression coefficient = -0.057 (95% CI = -0.740, - 0.038), p = 1.08 x 10-9(PDF 158 kb)
ESM Table 1
(PDF 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Downes, K., Marcovecchio, M.L., Clarke, P. et al. Plasma concentrations of soluble IL-2 receptor α (CD25) are increased in type 1 diabetes and associated with reduced C-peptide levels in young patients. Diabetologia 57, 366–372 (2014). https://doi.org/10.1007/s00125-013-3113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3113-8