Abstract
Proinflammatory cytokines released from the pancreatic islet immune cell infiltrate in type 1 diabetes (T1D) cause insulinopenia as a result of severe beta cell loss due to apoptosis. Diabetes prevention strategies targeting different cytokines with antibodies in combination with a T cell antibody, anti-TCR, have been assessed for therapy success in the LEW.1AR1-iddm (IDDM) rat, an animal model of human T1D. Immediately after diabetes manifestation, antibody combination therapies were initiated over 5 days with anti-TNF-α (tumour necrosis factor), anti-IL-1β (interleukin), or anti-IFN-γ (interferon) together with anti-TCR for the reversal of the diabetic metabolic state in the IDDM rat. Anti-TCR alone showed only a very limited therapy success with respect to a reduction of immune cell infiltration and beta cell mass regeneration. Anti-TCR combinations with anti-IL-1β or anti-IFN-γ were also not able to abolish the increased beta cell apoptosis rate and the activated immune cell infiltrate leading to a permanent beta cell loss. In contrast, all anti-TCR combinations with anti-TNF-α provided sustained therapy success over 60 to 360 days. The triple combination of anti-TCR with anti-TNF-α plus anti-IL-1β was most effective in regaining sustained normoglycaemia with an intact islet structure in a completely infiltration-free pancreas and with a normal beta cell mass. Besides the triple combination, the double antibody combination of anti-TCR with anti-TNF-α proved to be the most suited therapy for reversal of the T1D metabolic state due to effective beta cell regeneration in an infiltration free pancreas.
Key messages
-
Anti-TCR is a cornerstone in combination therapy for autoimmune diabetes reversal.
-
The combination of anti-TCR with anti-TNF-α was most effective in reversing islet immune cell infiltration.
-
Anti-TCR combined with anti-IL-1β was not effective in this respect.
-
The combination of anti-TCR with anti-TNF-α showed a sustained effect over 1 year.
Similar content being viewed by others
Introduction
New immunomodulatory intervention therapies to counteract beta cell loss due to type 1 diabetes (T1D) development must target proinflammatory cytokines released from activated immune cells to counteract their beta cell toxic potential [1,2,3,4]. It is general consensus nowadays that therapy success requires immunomodulatory combination therapies with different antibodies [5,6,7,8,9]. To be successful, these therapies have to target in particular the two main proinflammatory cytokines, namely IL (interleukin)-1β and TNF (tumour necrosis factor)-α, in the pancreatic islet immune cell infiltrate [1,2,3]. Successful combination therapies require in addition the inclusion of a T cell–specific antibody, as a cornerstone antibody, against the TCR/CD3 (T cell receptor/cluster of differentiation) complex, as documented in the T1D situation both in humans [10,11,12,13,14] and rat models of autoimmune diabetes [15, 16].
In order to identify a therapeutic antibody combination with maximal curative potential suitable for translation to the patient with newly diagnosed T1D, we studied in the present investigation in the IDDM (LEW.1AR1-iddm) rat, a model of human T1D [17,18,19], a variety of combinations composed of two or three therapeutic antibodies. The analyses of different combinations made it possible to identify antibody combinations with optimal curative potential ideally suited for translation to the patient with T1D in the early phase after diabetes manifestation in order to reverse the diabetic metabolic state and insulin deficiency due to the reconstitution of an infiltration-free endocrine pancreas along with a full regeneration of the beta cell mass.
Since the pancreatic beta cell is so extremely vulnerable due to its low protection against stress [20] along with a limited beta cell regeneration potential [21,22,23], intensive efforts are required to establish effective combination therapies with a strong capacity for suppression of destructive islet immune cell infiltration and a high potential for beta cell regeneration. This need for combination therapies with high curative potential in T1D is not so evident in other autoimmune diseases such as rheumatoid arthritis and inflammatory bowel diseases [10, 13, 24,25,26,27,28], where the more modest therapy goal, namely a symptom-free remission of the disease, can be reached in many cases also with an anti-cytokine monotherapy, such as with anti-TNF-α [25, 26, 29, 30].
Material and methods
Animals
Congenic LEW.1AR1-iddm (IDDM) rats (for details, see http://www.mh-hannover.de/34926.html) were bred and maintained under standard conditions in the Central Animal Facility of Hannover Medical School with viral and genetic monitoring [16, 17, 19]. Experimental procedures were approved by the District Government of Hannover (LAVES, no 33-42502-05/958 & 509.6-42502-03/684 and 33.9-42502-04/16/2115) in accordance with the guidelines for the care and use of laboratory animals.
Experimental groups
Different experimental groups with IDDM rats of both sexes were studied. Group 1 (n = 6) comprised healthy, normoglycaemic control rats; group 2 (n = 11) comprised diabetic rats treated for 5 consecutive days with anti-TCR alone (0.5 mg/kg b.wt. i.v.), an antibody directed against the α/β chains of the TCR/CD3 complex (R73, Serotec, Munich, Germany) [31]. Group 3 (n = 10) comprised diabetic rats treated for 5 consecutive days with anti-TCR with a rat-specific anti-IFN-γ (DB1, 0.1 mg/kg b.wt. i.v.) [32, 33]. Group 4 (n = 10) comprised diabetic rats treated for 5 consecutive days with anti-TCR and a rat-specific anti-IL-β (0.1 mg/kg b.wt. i.v., a custom-made monoclonal antibody with the epitope of silk6 clone [Eurogentec, Liege, Belgium]) [34]. Group 5 (n = 5) comprised diabetic rats treated with anti-TCR and anti-TNF-α (5 mg/kg b.wt. i.v.; kindly provided by Janssen Research & Development, Spring House, PA, USA) with a follow-up of 60 days and in group 6 (n = 6) with the same combination with a follow-up of 360 days. Group 7 (n = 9) comprised diabetic rats treated for 5 consecutive days with the triple combination of anti-TCR, anti-TNF-α, and anti-IL-1β. Group 8 (n = 6) comprised non-treated acutely diabetic control rats. No adverse events were observed in any treatment group. All therapies were started within 1 day after diabetes onset at blood glucose concentrations > 7.5 mmol/l with a disease manifestation age of around 60 days (between 50 and 80 days). Animals in groups 1 and 8 received injections of 0.9% NaCl solution.
Pancreatic tissue and serum processing
To identify the changes in islets, one pancreas biopsy was performed on the day of diabetes manifestation, immediately before the start of therapy, and another at the end of therapy as described previously [35]. The remaining pancreas comprising head and tail was collected for analyses 60 days and after anti-TCR combination with anti-TNF-α also 360 days (group 6) after the end of therapy including serum samples. Blood glucose concentrations were determined daily (Glucometer Elite®, Bayer, Leverkusen, Germany). Serum C-peptides were analysed with a rat-specific ELISA (Mercodia, Uppsala, Sweden) and serum cytokine protein concentrations with a multiplex immunoassay kit (Bio-Rad, Munich, Germany) [15, 16].
Morphological analyses
Pancreatic sections were stained either with the avidin-biotin-complex or double immunofluorescence technique with primary antibodies for beta and immune cells [2, 16, 35]. The antibodies recognised epitopes other than those targeted by the therapeutic antibodies summarized in Supplementary Table S1. A minimum of 1000 beta cells were counted in the proliferation and apoptosis analyses. Twenty to 25 islets in pancreas biopsies and 30–50 islets in the organ were studied during treatment in each animal [15, 34, 35]. The beta cell mass, stained by insulin and GLUT2 on serial sections, was calculated as described before [15, 16]. Analyses were performed using an Olympus microscope BX61 and for scanning BX61VS (Olympus, Hamburg, Germany) [15, 16, 35].
In situ reverse transcriptase-polymerase chain reaction
Gene expression changes in islets after the therapy-free interval were identified by in situ reverse transcriptase-polymerase chain reaction (in situ RT-PCR) analysis of pancreatic sections from all groups as described [15, 16, 35]. The used primer sequences are provided in Table S2.
Statistical analyses
Results are presented as mean values ± SEM. Comparisons between the different therapy groups and the healthy or diabetic controls were analysed with ANOVA followed by Dunnett’s test or Bonferroni’s test for multiple comparisons with the Prism 5 program (Graphpad Inc, San Diego, CA, USA). Significance was accepted at p < 0.05.
Results
Metabolic effects after anti-TCR combination therapies with cytokine antibodies
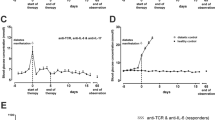
All therapies started within 1 day after diabetes manifestation (blood glucose > 7.5 mmol/l) in diabetic IDDM rats. Monotherapy with anti-TCR was given for 5 consecutive days (Fig. 1a). At most, transient instable glycaemia was achieved in very few of the treated diabetic rats (3/11) (Fig. 1a). Anti-TCR combined with either anti-IFN-γ, anti-IL-1β, or anti-TNF-α was administered for 5 consecutive days to the rats. With all three treatments, reductions of blood glucose values below 6 mmol/l were achieved in the presence of anti-TCR (Fig. 1b, c, e). However, a return to normoglycaemia from initial blood glucose values in the diabetic range between 8 and 15 mmol/l 60 days after the end of therapy was achieved only in less than 1/3 of the animals (3/10) treated with anti-IFN-γ (5.8 ± 0.2 mmol/l) (Fig. 1b) but in around 2/3 of the animals treated with anti-IL-1β (7/10) (5.6 ± 0.2 mmol/l) (Fig. 1c), and in all animals treated with anti-TNF-α (5/5) (5.2 ± 0.2 mmol/l) corresponding to healthy control animals (5.2 ± 0.2 mmol/l) (Fig. 1e).
Effects of a anti-TCR alone and anti-TCR combination therapies, b with anti-IFN-γ, or c anti-IL-1β or e anti-TNF-α over 60 days and f over 360 days, and g in a triple combination with anti-TNF-α plus anti-IL-1β on the metabolic profile of IDDM rats after diabetes manifestation compared to diabetic control rats (first column in all graphs with columns) without treatment and to healthy control rats (last column in all graphs with columns). a–c Blood glucose concentration (mmol/l) changes are shown for the responding and non-responding rats in response a to anti-TCR or the different anti-TCR combination therapies b with anti-IFN-γ, c anti-IL-1β or e anti-TNF-α for 60 days, f anti-TNF-α over 1 year and g in a triple combination anti-TNF-α plus anti-IL-1β. The first dashed line at day 0 indicates the start of therapy and the second dashed line at day 5 indicates the end of therapy. d, h Serum C-peptide concentration changes (pmol/l) are shown for rats responding and non-responding d to anti-TCR alone or the different combination therapies of anti-TCR with anti-IFN-γ or anti-IL-1β or h for rats responding and non-responding to the different combination therapies of anti-TCR with anti-TNF for 60 days after the end of therapy or over 1 year, or in the triple combination. Data are mean values ± SEM. Comparison of the different experimental groups including diabetic and healthy controls by one way ANOVA followed by the Bonferroni test. Diabetic control or each experimental group ***p < 0.001, **p < 0.01, or *p < 0.05 to the healthy control, $$$p < 0.001 to anti-TCR combination with anti-IFN-γ, §§§p < 0.001 to anti-TCR combination with anti-IL-1β, ^^^p < 0.001 to anti-TCR combination with anti-TNF-α 60 days, ∩∩∩p < 0.001 to anti-TCR combination with anti-TNF-α 360 days, ###p < 0.001 to anti-TCR combination with anti-TNF-α plus anti-IL-1β
In the successfully treated diabetic animals with the different anti-TCR combination therapies with anti-IFN-γ or anti-IL-1β, serum C-peptide concentrations doubled 60 days after the end of therapy compared with the values of the diabetic animals (200 ± 16 pmol/l) (first column) before the start of therapy or to the monotherapy with anti-TCR (356 ± 3 pmol/l) (Fig. 1d).
When animals were treated with anti-TCR and anti-TNF-α and kept either for 60 days or for a 1-year observation period (360 days after the end of therapy), therapy success could be maintained during this long observation period in 4 out of 6 rats (6.4 ± 0.5 mmol/l) (Fig. 1f) compared with a complete therapy success after the shorter period (5/5) (Fig. 1e).
Finally, a triple combination treatment for anti-IL-1β and anti-TNF-α with anti-TCR was tested. This was the most successful treatment combination with a mean blood glucose of 5.0 ± 0.2 mmol/l in 6 out of 9 rats at day 60 (Fig. 1g) being not significantly different from healthy control rats (last column).
In the successfully treated diabetic animals with the different anti-TCR combination therapies with anti-TNF-α, serum C-peptide concentrations were increased three- to fourfold 60 days after the end of therapy compared with the values of the rats in the diabetic control group (200 ± 16 pmol/l) (first column) before the start of therapy (Fig. 1h).
The different antibody therapies increased beta cell mass from initial values of around 2 mg in the diabetic control group before the start of therapy to values approaching 4 mg with the anti-TCR monotherapy (Fig. 2a) and those of the healthy control rats (around 6 mg) with distinct combination therapies (Fig. 2b–f). However, the efficiency of treatment differed between the different treatment groups reaching beta cell mass values above 5 mg at the end of the 60-day observation period (Fig. 2b–d, f). Interestingly, however, this therapy success could be achieved in the anti-TCR combination therapy with anti-IFN-γ typically only in animals with initial blood glucose concentration values below 12 mmol/l (Fig. 2b). In the combination therapy with anti-IL-1β, it was possible to regain beta cell mass values of around 5 mg even in half of the rats with starting blood glucose values above 12 mmol/l (Fig. 2c). Anti-TCR combination therapy with anti-TNF-α was most effective in terms of beta cell mass values in the range of 6 mg (Fig. 2d).
Relation between blood glucose concentration (mmol/l) at the start of therapy and beta cell mass (mg) a–c, d, f 60 days or e 1 year after the end of therapy in IDDM rats. a After anti-TCR alone and b after anti-TCR combination therapy with anti-IFN-γ, c with anti-IL-1β, d with anti-TNF-α all followed 60 days, e with anti-TNF-α followed for 1 year f in the triple combination with anti-TNF-α plus anti-IL-1β followed 60 days. The therapy success differed between the six analysed therapeutic groups either in the starting point of the blood glucose concentrations for the reversal to normoglycaemia or in the increase of beta cell mass, over 5 mg or around 6 mg representing the beta cell mass of healthy control animals
After a 1 year observation period in the animals treated with anti-TCR plus anti-TNF-α, the beta cell mass increase remained in the same range (Fig. 2e).
In the triple combination, all 6 animals with blood glucose values of up to 13 mmol/l showed a therapy success with a beta cell mass increase to around 6 mg (Fig. 2f).
Quantification of the effects of anti-TCR combination therapies with cytokine antibodies
Changes in beta cell proliferation and apoptosis rates
At the day of diabetes manifestation, immediately before the start of therapy, the diabetic rats that were responsive to the anti-TCR therapy alone or in combination with anti-IFN-γ or with anti-IL-1β showed a significant more than threefold increase of the proliferation rate analysed by Ki67 staining (Fig. 3a); the apoptosis rate increased more than 30-fold (Fig. 3b) compared with healthy controls. The proliferation rate in rats treated with anti-TCR and anti-TNF-α for 60 or 360 days or with the triple combination (Fig. 3e) showed also a threefold increase and the apoptosis rate a 20-fold increase (Fig. 3f). All these values before the start of therapy corresponded also to the values observed in diabetic control animals (Fig. 3a, b, e, f).
Morphometric analyses of beta cells and immune cells in IDDM rats after anti-TCR monotherapy and after successful anti-TCR combination therapy after diabetes manifestation with anti-IFN-γ, anti-IL-1β, and anti-TNF-α followed for 60 days after the end of therapy or with anti-TNF-α followed for 360 days, or combined with anti-IL-1β followed for 60 days after the end of therapy. Changes in the rate of a, e proliferation; b, f apoptosis; c, g islet infiltration score; and d, h pancreatic beta cell mass. Measurements were performed immediately before therapy, at the end of therapy, and 60 or 360 days after the end of therapy. Data are mean values ± SEM. Comparison of the different experimental groups including diabetic and healthy controls by one way ANOVA followed by the Bonferroni test. Diabetic control (first column in all graphs) or each experimental group ***p < 0.001, **p < 0.01, or *p < 0.05 to the healthy control (last column in all graphs). Σp < 0.05 to anti-TCR monotherapy, $$$p < 0.001 to anti-TCR combination with anti-IFN-γ, §§§p < 0.001 to anti-TCR combination with anti-IL-1β, ^^^p < 0.001 or ^^p < 0.01 to anti-TCR combination with anti-TNF-α 60 days, ∩∩∩p < 0.001 to anti-TCR combination with anti-TNF-α 360 days, ###p < 0.001or ##p < 0.01 to anti-TCR combination with anti-TNF-α plus anti-IL-1β
After the end of the monotherapy and combination therapies of anti-TCR with the different cytokine antibodies, the rates of proliferation (Fig. 3a, e) as well as of apoptosis (Fig. 3b, f) remained increased.
Even at 60 days after the end of therapy, in the responding rats with the combinations of anti-TCR plus anti-IFN-γ or anti-IL-1β, the proliferation rates (four to eightfold) as well as the apoptosis rates remained increased (20- till 30-fold) compared with those of healthy controls (Fig. 3a, b).
Only after the combination of anti-TCR with anti-TNF-α the proliferation rate (Fig. 3e) and apoptosis rate (Fig. 3f) returned to values in the range of the healthy control rats. The same was true for the proliferation and apoptosis rate after 1 year for this combination (Fig. 3e, f), documenting the stability of the therapeutic effect. An apoptosis rate in the normal range as an optimal result was achieved also with the triple antibody combination therapy (Fig. 3e, f).
Infiltration score
Before the start of therapy, the infiltration score of the islets was high with values of around 3 for all therapies (Fig. 3c, g). The insulitis score was marginally reduced immediately after the end of all combination therapies (Fig. 3c, g). At 60 days after the end of therapy, the infiltration score in the regenerated endocrine pancreases was only slightly reduced to values of around 2 for the anti-TCR monotherapy and the anti-TCR combination with anti-IFN-γ and to values of around 1 for the anti-TCR combination with anti-IL-1β (Fig. 3c). In all combination therapies of anti-TCR with anti-TNF-α including the combination plus anti-IL-1β, the infiltration score values were in the low range of the control values (0.1–0.3) independent from the observation period (60 or 360 days) (Fig. 3g).
Beta cell mass
After diabetes manifestation and before therapy, the beta cell mass was reduced in all diabetic rats to around 1/3 of the value in normoglycaemic healthy controls (Fig. 3d, h). Without treatment, these residual beta cells are lost within 1 week. Immediately after the end of anti-TCR alone or the anti-TCR combination therapies with the different cytokine antibodies, the respective beta cell mass remained low (Fig. 3d, h). The exception was the combination of anti-TCR with TNF-α, which showed already a clear beta cell mass increase immediately after the end of therapy (Fig. 3h).
Sixty days after the end of the different combination therapies, the beta cell mass had attained values approaching the normal range with the highest beta cell mass values comparable with healthy controls achieved in the combination therapies of anti-TCR with anti-TNF-α including the triple combination (Fig. 3h). In contrast, in the few partially successful combination therapies with anti-IL-1β and in particular with anti-IFN-γ beta cell mass increase was rather limited (Fig. 3d).
Islet immune cell infiltration pattern after anti-TCR combination therapies with cytokine antibodies
After diabetes manifestation, the islet infiltrate was composed of a high number of both immune cell types, CD8+ T cells and CD68 macrophages, and to a lesser extent of around 10 % CD4+ T cells as well as 10 % of NK-cells and B cells in the acutely diabetic rats (Fig. 4a).
Immune cell infiltration in pancreatic islets of IDDM rats after successful anti-TCR combination therapy with anti-IFN-γ, anti-IL-1β, or in the triple combination after diabetes manifestation and after the treatment-free period of 60 days after the end of therapy. a–d Beta cells (green) and immune cells (red) were examined in islets from animals successfully treated with the anti-TCR combination with b anti-IFN-γ, c anti-IL-1β, or d in the triple fashion anti-TCR combined together with anti-TNF-α plus anti-IL-1β after diabetes manifestation and compared with a the untreated diabetic situation. Islets were immunostained for insulin (green) and CD4+ T cells (red), CD8+ T cells (red), and CD68 macrophages (red) and counterstained with DAPI (blue). Erythrocytes were identified by yellow to orange colour through auto-fluorescence in the red and green channels. The quantification of these immune cells revealed for the healthy control only macrophages with the absolute number of 2 immune cells/islet, whereas the diabetic control showed 42 immune cells/islet with 4% CD4+ T cells, 45% CD8+ T cells, and 51% CD68 macrophages; anti-TCR combination with anti-IFN-γ reduced the immune cells to 16/islet with 5% CD4+ T cells, 43% CD8+ T cells and 53% CD68 macrophages. After anti-TCR therapy with anti-IL-1β the absolute number of immune cells was reduced to 8/islet with a predominance of 85 % macrophages. After anti-TCR combination with anti-TNF-α alone (60 and 360 days) or in the triple fashion with anti-IL-1β, only macrophages were present in the same amount as in the islets of healthy controls. Scale bar represents 25 μm
At 60 days after the end of the different anti-TCR combination therapies with anti-IFN-γ or anti-IL-1β, the responding rats showed still a significant residual immune cell infiltration in the islets (Fig. 4b, c). The same was true for the monotherapy with anti-TCR (not shown). After the triple anti-TCR combination therapy, however, no immune cell infiltration whatsoever was still present in the islets representing the situation in control islets (Fig. 4d).
Changes of cytokines in pancreatic islets and serum after anti-TCR combination therapies with cytokine antibodies
Gene expression
Compared to non-infiltrated islets from normoglycaemic control rats, high levels of gene expression of the proinflammatory cytokines, Ifng, Il1b, Tnf, and Il2 as well as the anti-inflammatory cytokines Il4 and Il10 were present in the infiltrating immune cells in the islets of diabetic rats (Table 1). Sixty days after the end of anti-TCR alone or of the anti-TCR combination therapy with anti-IFN-γ a marginal decrease and with anti-IL-1β, a moderate decrease was observed in the gene expression of proinflammatory cytokines in the immune cells, while the expression of anti-inflammatory cytokines was not significantly affected (Table 1). In contrast, the different combinations of anti-TCR with anti-TNF-α strongly reduced both the pro- and anti-inflammatory cytokine gene expression (Table 1).
In the triple combination therapy mode, pro- and anti-inflammatory cytokine gene expression was virtually completely abolished close to those values in islets of healthy controls (Table 1).
Protein expression
Sixty days after the end of combination therapies with all cytokine antibodies, the serum protein concentrations of the proinflammatory cytokines were significantly reduced in particular in the combinations with anti-IL-1β and anti-TNF-α, when compared with diabetic control animals (Fig. 5a–c). After the triple combination therapy, the serum protein concentrations of the three proinflammatory cytokines were low or even lower than those in healthy control animals (Fig. 5a–c).
Cytokine pattern in serum of IDDM rats after anti-TCR alone and after successful anti-TCR combination therapies with anti-IFN-γ, anti-IL-1β, and anti-TNF-α all followed for 60 days and with anti-TNF-α also for 1 year or in triple combination followed for 60 days after diabetes manifestation. Changes in protein concentrations of cytokines measured by the multiplex analysis were examined a IFN-γ, b IL-1β, c TNF-α, d IL-2, e IL-4, and f IL-10. Results after anti-TCR alone and anti-TCR combination therapy with anti-IFN-γ, with anti-IL-1β, with anti-TNF-α for 60 days or 1 year, or triple fashion were compared with those from healthy control rats (last column) and diabetic control rats without treatment (first column). Cytokine protein concentrations (pg/ml) are expressed as mean values ± SEM; a–f the dotted lines show changes in the proinflammatory and anti-inflammatory cytokines compared with the normoglycaemic control. Comparison of the different experimental groups including diabetic and healthy controls by one way ANOVA followed by the Bonferroni test. Diabetic control (first column in all graphs) or each experimental group ***p < 0.001, **p < 0.01, or *p < 0.05 versus healthy controls (last column in all graphs), Σp < 0.05 to anti-TCR monotherapy, $$$p < 0.001, $$p < 0.01, or $p < 0.05 to anti-TCR combination with anti-IFN-γ, §§§p < 0.001 or §p < 0.05 to anti-TCR combination with anti-IL-1β, ^^^p < 0.001 or ^^p < 0.01 to anti-TCR combination with anti-TNF-α 60 days, ∩∩∩p < 0.001, ∩∩p < 0.01 or ∩p < 0.05 to anti-TCR combination with anti-TNF-α 360 days, ###p < 0.001, ##p < 0.01, or #p < 0.05 to anti-TCR combination with anti-TNF-α plus anti-IL-1β
The high level of immune cell activating cytokine IL-2 decreased significantly in animals treated with the triple combination (Fig. 5d). The anti-inflammatory cytokines IL-4 and IL-10 which decreased in the diabetic control rats increased in animals treated with anti-TCR in combination with anti-IL-1β and anti-TNF-α (Fig. 5e, f).
The results document on the gene and protein level of pancreatic islets and serum cytokines a clear superiority of anti-TCR combinations with anti-TNF-α in all parameters over those with anti-IFN-γ or anti-IL-1β and with a further therapy optimization in the case of the triple antibody combination.
Discussion
Successful therapy of T1D is dependent upon the administration of a CD3/TCR antibody to reverse the activation of the T cells in the immune cell infiltrate in the endocrine pancreas [12, 14, 24]. In addition, proinflammatory cytokines produced by different infiltrating immune cells play a crucial role in the pathogenesis of this autoimmune disease. In concert, the toxic action of the T cells and the proinflammatory cytokines mediate beta cell destruction in the islets of Langerhans, thereby causing an insulinopenia which results in the development of diabetic hyperglycaemia [3, 36,37,38]. Targeting single components of the autoimmune disease process did not yield sustained therapy success [5, 39]. It is therefore consensus in the scientific community that combination therapies are required to target the different components of the disease process and thereby reverse diabetic hyperglycaemia through suppression of the autoimmune-mediated beta cell destructive process and the subsequent regeneration of the insulin-producing cells [6,7,8,9].
The aim of this study was to device combination therapies with maximal curative potential in the IDDM rat, an animal model of autoimmune diabetes, which mirrors the human T1D situation optimally [2, 17, 19, 40]. The model is thus very much suited for the transferal of curative combination therapy concepts to patients with T1D in the early phase of the disease immediately after the manifestation of diabetic hyperglycaemia.
The results show that the combined administration of an antibody against TCR/CD3 and an antibody against TNF-α is the minimum requirement for achieving a long-term therapy success, as documented by a sustained suppression of autoimmunity in connection with the abolishment of the beta cell toxic effect of the proinflammatory cytokine TNF-α. Only this combination returned beta cell proliferation and apoptosis rates into the low range of healthy animals. The combination of anti-TCR with anti-IL-1β did not provide this sustained effect and a combination of anti-TCR with anti-IFN-γ was even more ineffective in the same way as the monotherapy with anti-TCR or anti-CD3 [13, 15, 41], thereby limiting the recovery of the beta cell mass. This explains also why the scientists of the Trial Network call this monotherapy a “disease-modifying therapy” [42] without curative potential. Even when these therapies showed a certain reduction of hyperglycaemia, only the combination of anti-TCR with anti-TNF-α provided this desired sustained curative effect, documented by a regain of an infiltration-free pancreas with sufficient beta cell mass along with a sustained fully normalized blood glucose profile.
The message from the present studies is therefore that a reduction of hyperglycaemia should not be taken as the sole indicator for therapy success, as it is often done in clinical studies, since this is not a valid parameter for proving curative therapy success.
Interestingly, the triple combination turned out to be the most effective therapeutic antibody combination yielding an optimal result with regenerated beta cells in the islets fully resembling in their structure healthy non-immune cell-infiltrated control islets. It was also the only therapy scheme that reduced all serum cytokines to levels below those of healthy control animals.
The key message from these studies with different combinations of antibodies is that for maximal therapy success, it is crucial to target the activated T cells with anti-TCR plus a targeting of the proinflammatory cytokines TNF-α and IL-1β with specific antibodies. Remarkable is in this context that this optimal therapy success was achieved with low doses of each of the antibodies in the combination, thereby minimizing the risk of serious side effects of this neutralizing immunomodulatory antibody therapy [24, 43]. Importantly, all these antibodies are also available as humanised antibodies and established in the therapies of a number of autoimmune diseases besides T1D such as rheumatoid arthritis and inflammatory bowel diseases [10, 13, 24,25,26,27,28]. Interestingly, antibodies against IFN-γ are also not established in the therapy of any other autoimmune disease [9, 44].
This combination therapy of anti-TCR with anti-TNF-α is a viable option especially in the LADA (latent autoimmune diabetes in adults) form of autoimmune diabetes in view of the slower disease progression both in LADA patients and in a rat model of human LADA than in patients and in the IDDM rat model with early manifesting T1D [36, 40, 45,46,47,48].
On the other hand, the triple combination as the most quickly acting and most effective combination therapy form should be the preferred treatment in all cases where the beta cell mass is already strongly reduced (< 30–40% of normal) and thus at the limit where it is still possible to initiate successfully a regeneration process of the residual beta cells strong enough for a regain of a functional beta cell mass required for restitution of normoglycaemia. This is likely to be the case when patients develop the disease at an early age. As it is the only combination which abolishes both the expression of TNF-α and IL-1β thereby maximally suppressing the beta cell destructive potential of the proinflammatory cytokines, it is optimally suited not only to prevent progression of beta cell loss but also the regain of a normal beta cell mass. This is a very urgent need in particular in children manifesting T1D, in whom beta cell loss is particularly quickly progressing. On the other hand, even the most effective therapy scheme did not yield the desired curative therapy results, when the residual beta cell mass was reduced by more than 70%, as documented in the present study in the rats that had blood glucose concentrations at therapy start in the range between 15 and 20 mmol/l. This documents the need for early therapy start also in T1D.
Data availability
The datasets generated during and/or analysed during the current study are available from the author A.J. upon reasonable questions.
Change history
01 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00109-021-02151-6
Abbreviations
- anti-TCR antibody:
-
Anti–T cell receptor antibody
- Casp3 (Cpp32):
-
Caspase 3
- IDDM rat :
-
LEW.1AR1-iddm rat
- IFN-γ:
-
Interferon-gamma
- IL-1β:
-
Interleukin-1beta
- TCR/CD3 complex:
-
T cell receptor/cluster of differentiation complex
- T1D:
-
Type 1 diabetes
- TNF-α:
-
Tumour necrosis factor-alpha
References
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383(9911):69–82
Jörns A, Arndt T, Meyer zu Vilsendorf A, Klempnauer J, Wedekind D, Hedrich HJ, Marselli L, Marchetti P, Harada N, Nakaya Y et al (2014) Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia 57(3):512–521
Knight RR, Kronenberg D, Zhao M, Huang GC, Eichmann M, Bulek A, Wooldridge L, Cole DK, Sewell AK, Peakman M et al (2013) Human beta-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes 62(1):205–213
Faustman DL, Davis M (2009) The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J Mol Med (Berl) 87(12):1173–1178
Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M (2019) The challenge of modulating beta-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 7(1):52–64
Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M (2010) Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 160(2):176–184
Ryden AK, Wesley JD, Coppieters KT, Von Herrath MG (2014) Non-antigenic and antigenic interventions in type 1 diabetes. Hum Vaccin Immunother 10(4):838–846
Ludvigsson J (2014) Combination therapy for preservation of beta cell function in type 1 diabetes: new attitudes and strategies are needed! Immunol Lett 159(1-2):30–35
Nepom GT, Ehlers M, Mandrup-Poulsen T (2013) Anti-cytokine therapies in T1D: concepts and strategies. Clin Immunol 149(3):279–285
Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC et al (2013) Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes 62(11):3901–3908
MacDonald A, Ambery P, Donaldson J, Hicks K, Keymeulen B, Parkin J (2016) Subcutaneous administration of otelixizumab is limited by injection site reactions: results of an exploratory study in type 1 diabetes mellitus patients. Exp Clin Endocrinol Diabetes 124(5):288–293
Chatenoud L (2019) A future for CD3 antibodies in immunotherapy of type 1 diabetes. Diabetologia 62(4):578–581
Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C et al (2010) Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 53(4):614–623
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS et al (2019) An anti-CD3 antibody, Teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381:603–613
Jörns A, Akin M, Arndt T, Terbish T, Meier zu Vilsendorf A, Wedekind D, Hedrich HJ, Lenzen S (2014) Anti-TCR therapy combined with fingolimod for reversal of diabetic hyperglycemia by beta cell regeneration in the LEW.1AR1-iddm rat model of type 1 diabetes. J Mol Med (Berl) 92(7):743–755
Jörns A, Ertekin ÜG, Arndt T, Terbish T, Wedekind D, Lenzen S (2015) TNF-alpha antibody therapy in combination with the T-cell specific antibody anti-TCR reverses the diabetic metabolic state in the LEW.1AR1-iddm rat. Diabetes 64:2880–2891
Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S (2005) Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes 54(7):2041–2052
Lenzen S (2017) Animal models of human type 1 diabetes for evaluating combination therapies and successful translation to the patient with type 1 diabetes. Diabetes Metab Res Rev 33(7):e2915
Lenzen S, Tiedge M, Elsner M, Lortz S, Weiss H, Jörns A, Klöppel G, Wedekind D, Prokop CM, Hedrich HJ (2001) The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia 44(9):1189–1196
Lenzen S (2008) Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 36(3):343–347
Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57(6):1584–1594
Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC (2013) Beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 36(1):111–117
Bakhti M, Bottcher A, Lickert H (2018) Modelling the endocrine pancreas in health and disease. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-018-0132-z
Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W et al (2019) Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62(4):655–664
Kalden JR (2015) Pathogenic cells of rheumatic inflammation as the target of modern therapies. Z Rheumatol 74(1):8–13
Ordas I, Mould DR, Feagan BG, Sandborn WJ (2012) Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 91(4):635–646
Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM (2018) Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol 71(21):2392–2401
Seelig E, Timper K, Falconnier C, Stoeckli R, Bilz S, Oram R, McDonald TJ, Donath MY (2016) Interleukin-1 antagonism in type 1 diabetes of long duration. Diabetes Metab 42(6):453–456
Menegatti S, Bianchi E, Rogge L (2019) Anti-TNF therapy in spondyloarthritis and related diseases, impact on the immune system and prediction of treatment responses. Front Immunol 10:382
Lenzen H, Qian J, Manns MP, Seidler U, Jörns A (2018) Restoration of mucosal integrity and epithelial transport function by concomitant anti-TNFalpha treatment in chronic DSS-induced colitis. J Mol Med (Berl) 96(8):831–843
Hünig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G (1989) A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med 169(1):73–86
Rosenberg AS, Finbloom DS, Maniero TG, van der Meide PH, Singer A (1990) Specific prolongation of MHC class II disparate skin allografts by in vivo administration of anti-IFN-gamma monoclonal antibody. J Immunol 144(12):4648–4650
Voorthuis JA, Uitdehaag BM, De Groot CJ, Goede PH, van der Meide PH, Dijkstra CD (1990) Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol 81(2):183–188
Aharon-Hananel G, Jörns A, Lenzen S, Raz I, Weksler-Zangen S (2015) Antidiabetic effect of interleukin-1beta antibody therapy through beta-cell protection in the Cohen diabetes-sensitive rat. Diabetes 64(5):1780–1785
Jörns A, Rath KJ, Terbish T, Arndt T, Meyer zu Vilsendorf A, Wedekind D, Hedrich HJ, Lenzen S (2010) Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology 151(8):3555–3565
Skog O, Korsgren O (2018) Aetiology of type 1 diabetes: physiological growth in children affects disease progression. Diabetes Obes Metab 20(4):775–785
Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M (2015) Current concepts on the pathogenesis of type 1 diabetes-considerations for attempts to prevent and reverse the disease. Diabetes Care 38(6):979–988
Arnush M, Scarim AL, Heitmeier MR, Kelly CB, Corbett JA (1998) Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160(6):2684–2691
Greenbaum CJ, Speake C, Krischer J, Buckner J, Gottlieb PA, Schatz DA, Herold KC, Atkinson MA (2018) Strength in numbers: opportunities for enhancing the development of effective treatments for type 1 diabetes-the TrialNet experience. Diabetes 67(7):1216–1225
Lenzen S, Arndt T, Elsner M, Wedekind D, Jörns A (2020) Rat models of human diabetes. Animal models of diabetes: Methods and Protocols. Methods Mol Biol 2128(Editor: Aileen King; Publisher Springer Nature):69–85
Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, Galleri L, Spagnuolo I, Steidler L, Van Huynegem K et al (2012) Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122(5):1717–1725
Bingley PJ, Wherrett DK, Shultz A, Rafkin LE, Atkinson MA, Greenbaum CJ (2018) Type 1 Diabetes TrialNet: a multifaceted approach to bringing disease-modifying therapy to clinical use in type 1 diabetes. Diabetes Care 41(4):653–661
Davis IC, Randell J, Davis SN (2015) Immunotherapies currently in development for the treatment of type 1 diabetes. Expert Opin Investig Drugs 24(10):1331–1341
Chasset F, Arnaud L (2018) Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev 17(1):44–52
Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD et al (2012) Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes 61(8):2066–2073
Madsbad S, Faber OK, Binder C, McNair P, Christiansen C, Transbol I (1978) Prevalence of residual beta-cell function in insulin-dependent diabetics in relation to age at onset and duration of diabetes. Diabetes 27(Suppl 1):262–264
Oram RA, McDonald TJ, Shields BM, Hudson MM, Shepherd MH, Hammersley S, Pearson ER, Hattersley AT, Team U (2015) Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 38(2):323–328
Jörns A, Wedekind D, Jähne J, Lenzen S (2020) Pancreas pathology of latent autoimmune diabetes in adults (LADA) in patients and in a LADA rat model compared to type 1 diabetes mellitus. Diabetes 69(4):624–633
Acknowledgements
S.Y., D.I., and T.Y. were on leave from the Department of Digestive Surgery and Transplantation, University of Tokushima, Tokushima, Japan. We thank D. Lischke and R. Chucholl, both Institute of Clinical Biochemistry, Hannover Medical School, Germany, for skillful technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work has been supported by a grant from the Deutsche Forschungsgemeinschaft (JO 395/2-2).
Author information
Authors and Affiliations
Contributions
A.J. designed the study, performed experiments, analysed and interpreted data, and wrote the manuscript. T.A., D.I., S.Y., T.Y., and T.T. performed experiments. D. W. and P.H.M. provided materials and reviewed the manuscript. S.L. designed the study, analysed and interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jörns, A., Arndt, T., Yamada, S. et al. Translation of curative therapy concepts with T cell and cytokine antibody combinations for type 1 diabetes reversal in the IDDM rat. J Mol Med 98, 1125–1137 (2020). https://doi.org/10.1007/s00109-020-01941-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01941-8