Abstract
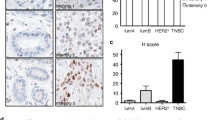
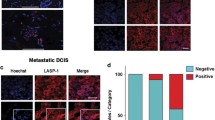
LRP1B intracellular domain is released and transported to the nucleus; however, pathological consequences of this nuclear transport are largely unclear. We aimed to unravel the pathobiological significance of nuclear localization of LRP1B intracellular domain in mammary gland carcinogenesis. Immunohistochemical staining using antibodies for LRP1B intracellular domain was performed to determine LRP1B expression in 92 invasive ductal breast carcinomas. LRP1B immunoreactivity was detected in the surface membrane and cytoplasm of 60 of 92 invasive ductal carcinomas and in the nucleus of 15 of 92 carcinomas. Nuclear LRP1B was significantly associated with poor patient prognosis, particularly luminal A type breast cancer, where it was significantly related to nodal metastasis. Doxycycline-dependent nuclear expression of LRP1B intracellular domain was established in cultured breast cancer cells. Enforced nuclear expression significantly increased Matrigel invasion activity in MCF-7 and T47D luminal A breast cancer cells. Moreover, enforced nuclear expression of LRP1B intracellular domain facilitated MCF-7 cells growth in mammary fat pad of nude mice, which was supplemented with estrogen. Comprehensive microarray-based analysis demonstrated that nuclear expression of LRP1B intracellular domain significantly increased long non-coding RNA nuclear paraspeckle assembly transcript 1 (NEAT1) expression, which facilitates breast cancer invasion with poor prognosis. Nuclear-localized LRP1B intracellular domain promoted breast cancer progression with poor prognosis, possibly through the NEAT1 pathway. Nuclear transport of LRP1B intracellular domain could be a therapeutic target for breast cancer patients.
Key messages
-
Nuclear LRP1B was significantly associated with poor patient prognosis.
-
Nuclear LRP1B increased Matrigel invasion activity of breast cancer cells.
-
Nuclear expression of LRP1B intracellular domain increased NEAT1 expression.







Similar content being viewed by others
References
Go GW, Mani A (2012) Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med 85:19–28
Willnow TE, Nykjaer A, Herz J (1999) Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol 1:E157–E162
Liu CX, Li Y, Obermoeller-McCormick LM, Schwartz AL, Bu G (2001) The putative tumor suppressor LRP1B, a novel member of the low density lipoprotein (LDL) receptor family, exhibits both overlapping and distinct properties with the LDL receptor-related protein. J Biol Chem 276:28889–28896
Liu CX, Musco S, Lisitsina NM, Forgacs E, Minna JD, Lisitsyn NA (2000) LRP-DIT, a putative endocytic receptor gene, is frequently inactivated in non-small cell lung cancer cell lines. Cancer Res 60:1961–1967
Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455:1069–1075
Langbein S, Szakacs O, Wilhelm M, Sukosd F, Weber S, Jauch A, Lopez Beltran A, Alken P, Kälble T, Kovacs G (2002) Alteration of the LRP1B gene region is associated with high grade of urothelial cancer. Lab Investig 82:639–643
Sonoda I, Imoto I, Inoue J, Shibata T, Shimada Y, Chin K, Imamura M, Amagasa T, Gray JW, Hirohashi S, Inazawa J (2004) Frequent silencing of low density lipoprotein receptor-related protein 1B (LRP1B) expression by genetic and epigenetic mechanisms in esophageal squamous cell carcinoma. Cancer Res 64:3741–3747
Nakagawa T, Pimkhaokham A, Suzuki E, Omura K, Inazawa J, Imoto I (2006) Genetic or epigenetic silencing of low density lipoprotein receptor-related protein 1B expression in oral squamous cell carcinoma. Cancer Sci 97:1070–1074
Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C, Harshman K, Guipponi M, Bukach O, Zoete V, Michielin O, Muehlethaler K, Speiser D, Beckmann JS, Xenarios I, Halazonetis TD, Jongeneel CV, Stevenson BJ, Antonarakis SE (2011) Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat Genet 44:133–139
Beer AG, Zenzmaier C, Schreinlechner M, Haas J, Dietrich MF, Herz J, Marschang P (2016) Expression of a recombinant full-length LRP1B receptor in human non-small cell lung cancer cells confirms the postulated growth-suppressing function of this large LDL receptor family member. Oncotarget 7:68721–68733
Wang Z, Sun P, Gao C, Chen J, Li J, Chen Z, Xu M, Shao J, Zhang Y, Xie J (2017) Down-regulation of LRP1B in colon cancer promoted the growth and migration of cancer cells. Exp Cell Res 357:1–8
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499:214–218
An O, Pendino V, D'Antonio M, Ratti E, Gentilini M, Ciccarelli FD (2014) NCG 4.0: the network of cancer genes in the era of massive mutational screenings of cancer genomes. Database (Oxford) 2014:bau015
Lynn M, Shah N, Conroy J, Ennis S, Morris T, Betts D, O'Sullivan M (2014) A study of alveolar rhabdomyosarcoma copy number alterations by single nucleotide polymorphism analysis. Appl Immunohistochem Mol Morphol 22:213–221
Brown MS, Ye J, Rawson RB, Goldstein JL (2000) Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 2000(100):391–398
May P, Reddy YK, Herz J (2002) Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem 277:18736–18743
Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D (2004) Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279:34302–34310
Mi K, Johnson GV (2007) Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem 101:517–529
Polavarapu R, An J, Zhang C, Yepes M (2008) Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol 172:1355–1362
Li Y, Cong R, Biemesderfer D (2008) The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 295:C529–C537
Liu CX, Ranganathan S, Robinson S, Strickland DK (2007) Gamma-secretase-mediated release of the low density lipoprotein receptor-related protein 1B intracellular domain suppresses anchorage-independent growth of neuroglioma cells. J Biol Chem 282:7504–7511
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y (2000) Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem 74:1489–1497
Takeuchi T, Adachi Y, Nagayama T (2012) WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis 33:548–554
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
Bunai K, Okubo H, Hano K, Inoue K, Kito Y, Saigo C, Shibata T, Takeuchi T (2018) TMEM207 hinders the tumour suppressor function of WWOX in oral squamous cell carcinoma. J Cell Mol Med 22:1026–1033
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422:438–441
Zhang J, Zhao B, Chen X, Wang Z, Xu H, Huang B (2018) Silence of long noncoding RNA NEAT1 inhibits malignant biological behaviors and chemotherapy resistance in gastric Cancer. Pathol Oncol Res 24:109–113
Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA (2003) Nuclear Association of the Cytoplasmic Tail of MUC1 and β-catenin. J Biol Chem 278:38029–38039
Zhang L, Song X, Wang X, Xie Y, Wang Z, Xu Y, You X, Liang Z, Cao H (2015) Circulating DNA of HOTAIR in serum is a novel biomarker for breast cancer. Breast Cancer Res Treat 152:199–208
Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC (2017) Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer 16:104
Haas J, Beer AG, Widschwendter P, Oberdanner J, Salzmann K, Sarg B, Lindner H, Herz J, Patsch JR, Marschang P (2011) LRP1b shows restricted expression in human tissues and binds to several extracellular ligands, including fibrinogen and apoE-carrying lipoproteins. Atherosclerosis 216:342–347
Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK (2006) Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J 25:1242–1252
Musgrove EA, Sutherland RL (2009) Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9:631–643
Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M, MacDonald TY, Fontugne J, Erho N, Vergara IA, Ghadessi M, Davicioni E, Jenkins RB, Palanisamy N, Chen Z, Nakagawa S, Hirose T, Bander NH, Beltran H, Fox AH, Elemento O, Rubin MA (2014) The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5:5383
Li W, Zhang Z, Liu X, Cheng X, Zhang Y, Han X, Zhang Y, Liu S, Yang J, Xu B, He L, Sun L, Liang J, Shang Y (2017) The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest 127:3421–3440
Funding
This study was supported by grants from the Ministry of Education of Japan (grant nos. KAKEN 15K08361, 15K19051, and 17K15642).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Paraffin-embedded tissues surgically resected from patients with breast cancer were retrospectively used after diagnosis. The need for written informed consent was waived by the Institutional Review Board of the Gifu University Graduate School of Medicine. Instead, the Institutional Review Board requested us to inform the patients that they could refuse the use of their tissue specimens for this study if they did not want to participate in the present study. The experimental protocol to obtain the antibodies was approved by the Animal Care Committee of Gifu University. The present study was conducted in accordance with the ethical standards of the Helsinki Declaration in 1975, after approval of the Institutional Review Board of the Gifu University Graduate School of Medicine (specific approval number 25–81).
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Suppl File 1
Doxycycline treatment did not affect endogenous LRP1B mRNA transcript expression in MCF-7 and T47D cells. The value for the groups (n = 3) was calculated as the fold-change relative to the mean value for the control non-treated group (control set to 1.0). Standard deviations were then computed for the triplicate sets. Three target genes and fold changes are presented. Student’s t test was performed to determine the significant differences. P = 0.93 (24 h), P = 0.86 (48 h), P = 0.79 (72 h), and P = 0.76 (96 h) in MCF-7 cells. P = 0.15 (24 h), P = 0.80 (48 h), P = 0.66 (72 h), and P = 0.34 (96 h) in T47D cells. (PNG 149 kb)
Suppl File 2
Doxycycline-controlled expression of LRP1B intracellular domain increased MUC1 expression but did not increased nuclear MUC1-CTD in MCF-7 cells. Expression of LRP1B intracellular domain was induced by doxycycline at 100 ng/mL (indicated as Tet-on), and without doxycycline (indicated as Tet-off). Note the marked diminishment of MUC1 immunoreactivity in MUC1-targeted siRNA treated cells. (PNG 15 kb)
Suppl File 3
A: Both MUC1 targeting siRNAs markedly impaired expression of MUC1 and MUC1-CTD protein. B: Down-regulation of MUC1 did not alter the expression of MALAT1 or NEAT1. C: Down-regulation MUC1 did not alter the Matrigel invasion activity in LRP1B intracellular domain increased MCF-7 cells (PNG 624 kb)
Rights and permissions
About this article
Cite this article
Asano, Y., Takeuchi, T., Okubo, H. et al. Nuclear localization of LDL receptor-related protein 1B in mammary gland carcinogenesis. J Mol Med 97, 257–268 (2019). https://doi.org/10.1007/s00109-018-01732-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-01732-2