Abstract
Purpose
To appraise the ability of a radiomics signature to predict clinical outcome after definitive radiochemotherapy (RCT) of stage III–IV head and neck cancer.
Methods
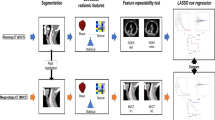
A cohort of 110 patients was included in a retrospective analysis. Radiomics texture features were extracted from the gross tumor volumes contoured on planning computed tomography (CT) images. The cohort of patients was randomly divided into a training (70 patients) and a validation (40 patients) cohorts. Textural features were correlated to survival and control data to build predictive models. All the significant predictors of the univariate analysis were included in a multivariate model. The quality of the models was appraised by means of the concordance index (CI).
Results
A signature with 3 features was identified as predictive of overall survival (OS) with CI = 0.88 and 0.90 for the training and validation cohorts, respectively. A signature with 2 features was identified for progression-free survival (PFS; CI = 0.72 and 0.80); 2 features also characterized the signature for local control (LC; CI = 0.72 and 0.82). In all cases, the stratification in high- and low-risk groups for the training and validation cohorts led to significant differences in the actuarial curves. In the validation cohort the mean OS times (in months) were 78.9 ± 2.1 vs 67.4 ± 6.0 in the low- and high-risk groups, respectively, the PFS was 73.1 ± 3.7 and 50.7 ± 7.2, while the LC was 78.7 ± 2.1 and 63.9 ± 6.5.
Conclusion
CT-based radiomic signatures that correlate with survival and control after RCT were identified and allow low- and high-risk groups of patients to be identified.
Zusammenfassung
Zielsetzung
Ziel war die Untersuchung der Möglichkeit, mittels einer Radiomics-Signatur klinische Ergebnisse nach definitiver Radiochemotherapie (RCT) von Kopf-Hals-Tumoren im Stadium II–IV vorherzusagen.
Methoden
Eine Kohorte von 110 Patienten wurde in eine retrospektive Analyse einbezogen. Die Radiomics-Texturmerkmale wurden aus den Gross-Tumor-Volume-Konturen der Planungs-CT-Aufnahmen extrahiert. Die Patientengruppe wurde nach dem Zufallsprinzip in eine Trainingskohorte (70 Patienten) und eine Validierungskohorte (40 Patienten) unterteilt. Die Strukturmerkmale wurden mit den Überlebens- und Kontrolldaten korreliert, um Vorhersagemodelle zu erstellen. Alle signifikanten Prädiktoren der univariaten Analyse wurden in ein multivariates Modell aufgenommen. Die Qualität der Modelle wurde anhand des Konkordanzindex (CI) beurteilt.
Ergebnisse
Eine Signatur mit 3 Merkmalen wurde als prädiktiv für das Gesamtüberleben, OS, identifiziert, mit einem CI = 0,88 bzw. 0,90 für die Trainings- bzw. Validierungskohorte. Eine Signatur mit 2 Merkmalen wurde für das progressionsfreie Überleben, PFS, identifiziert (CI = 0,72 bzw. 0,80). Ebenfalls 2 Merkmale charakterisierten die Signatur für die lokale Kontrolle, LC (CI = 0,72 und 0,82). In allen Fällen führt die Stratifizierung in Hoch- und Niedrigrisikogruppen für die Trainings- und Validierungskohorte zu signifikanten Unterschieden in den aktuariellen Überlebenskurven. In der Validierungskohorte betrug die mittlere Überlebenszeit (in Monaten) 78,9 ± 2,1 gegenüber 67,4 ± 6,0 in der Niedrig- bzw. Hochrisikogruppe. Das progressionsfreie Überleben betrug 73,1 ± 3,7 gegenüber 50,7 ± 7,2; bei lokaler Kontrolle von 78,7 ± 2,1 gegenüber 63,9 ± 6,5.
Schlussfolgerung
Es wurden CT-basierte radiomische Signaturen identifiziert, die mit dem Überleben und der Kontrolle nach RCT korrelieren und es somit ermöglichen, Patienten mit niedrigem und hohem Risiko zu identifizieren.





Similar content being viewed by others
References
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout R, Granton P et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Lambin P, van Stiphout R, Starmans M et al (2013) Predicting outcomes in radiation oncology, multifactorial decision support systems. Nat Rev Clin Oncol 10:27–40
Aerts H, Velazquez E, Leijenaar R, Parmar C, Grossmann P, Carvalho S et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Vallières M, Kay-Rivest E, Perrin L, Liem X, Furstoss C, Aerts H et al (2017) Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci Rep 7:10117
Bogowicz M, Riesterer O, Ikenberg K, Stieb S, Moch H, Studer G et al (2017) Computed Tomography Radiomics predicts HPV status and local tumor control after definitive Radiochemotherapy in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 99:921–928
Bogowicz M, Leijenaar R, Tanadini-Lang S, Riesterer O, Pruschy M, Studer G et al (2017) Post-radiochemotherapy PET radiomics in head and neck cancer—the influence of radiomics implementation on the reproducibility of local control tumor models. Radiother Oncol 125:385–391
Bogowicz M, Riesterer O, Stark LS, Studer G, Unkelbach J, Guckenberger M, Tanadini-Lang S (2017) Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol 56:1531–1536
M. D. Anderson Cancer Center Head and Neck Quantitative Imaging Working Group (2018) Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients. Sci Rep 8:1524
Ren J, Tian J, Yuan Y, Dong D, Li X, Shi Y, Tao X (2018) Magnetic resonance imaging based radiomics signature for the preoperative discrimination of stage I–II and III–IV head and neck squamous cell carcinoma. Eur J Radiol 106:1–6
Leijenaar R, Bogowicz M, Jochems A, Hoebers FJ, Wesseling F, Huang S et al (2018) Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol 91:20170498
Ou D, Blanchard P, Rosellini S, Levy A, Nguyen F, Leijenaar R et al (2017) Predictive and prognostic value of CT based radiomics signature in locally advanced head and neck cancers patients treated with concurrent chemoradiotherapy or bioradiotherapy and its added value to Human Papillomavirus status. Oral Oncol 71:150–155
Cozzi L, Comito T, Fogliata A, Franzese C, Franceschini D, Bonifacio C et al (2019) Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS ONE 14:e210758
Kirienko M, Cozzi L, Antonovic L, Lozza L, Fogliata A, Voulaz E et al (2018) Prediction of disease free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging 45:207–271
Cozzi L, Dinapoli N, Fogliata A, Hsu W, Reggiori G, Lobefalo F et al (2017) Radiomics based analysis to predict local control and survival in hepatocellular carcinoma patients treated with volumetric modulated arc therapy. BMC Cancer 17:829
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C et al (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789
Sollini M, Cozzi L, Antunovic L, Chiti A, Kirienko M (2017) PET Radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep 7:358
Collins G, Reitsma J, Altman D, Moons K (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br Med J 350:g7594
R Core Team (2018) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed: 1 Jan 2019
Gabryś H, Buettner F, Sterzing F, Hauswald H, Bangert M (2018) Design and selection of machine learning methods using Radiomics and Dosiomics for normal tissue complication probability modeling of Xerostomia. Front Oncol 8:35
Riegel A, Berson A, Destian S et al (2006) Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys 65:726–732
Pavic M, Bogowicz M, Würms X, Glatz S, Finazzi T, Riesterer O et al (2018) Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol 57:1070–1074
Ger R, Craft D, Mackin D, Zhou S, Layman R, Jones AK et al (2018) Practical guidelines for handling head and neck computed tomography artifacts for quantitative image analysis. Comput Med Imaging Graph 69:134–139
Bagher-Ebadian H, Siddiqui F, Liu C, Movsas B, Chetty I (2017) On the impact of smoothing and noise on robustness of CT and CBCT radiomics features for patients with head and neck cancers. Med Phys 44:1755–1770
Parmar C, Grossmann P, Rietveld D, Rietbergen M, Lambin P, Aerts H (2015) Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front Oncol 5:272
Parmar C, Leijenaar R, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, Rietbergen M, Haibe-Kains B, Lambin P, Aerts H (2015) Radiomic feature clusters and prognostic signatures specific for lung and head & neck cancer. Sci Rep 5:11044
Biondi M, Vanzi E, De Otto G, Carbone SF, Nardone V, Banci Buonamici F (2018) Effects of CT FOV displacement and acquisition parameters variation on texture analysis features. Phys Med Biol 63:235021
Caramella C, Allorant A, Orlhac F, Bidault F, Asselain B, Ammari S, Jaranowski P, Moussier A, Balleyguier C, Lassau N, Pitre-Champagnat S (2018) Can we trust the calculation of texture indices of CT images? A phantom study. Med Phys 45:1529–1536
MICCAI/M.D. Anderson Cancer Center Head and Neck Quantitative Imaging Working Group (2017) Matched computed tomography segmentation and demographic data for oropharyngeal cancer radiomics challenges. Sci Data 4:170077
Elhalawani H, Lin T, Volpe S, Mohamed A, White AL, Zafereo J et al (2018) Machine learning applications in head and neck radiation oncology: lessons from open-source Radiomics challenges. Front Oncol 8:294
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Cozzi acts as a scientific advisor to Varian Medical Systems and is a Clinical Research Scientist at Humanitas Cancer Center. C. Franzese, A. Fogliata, D. Franceschini, P. Navarria, S. Tomatis and M. Scorsetti declare that they have no competing interests.
Additional information
The authors L. Cozzi and C. Franzese contributed equally to the manuscript.
Caption Electronic Supplementary Material
66_2019_1483_MOESM1_ESM.docx
The complementary materials contains low and high risk stratification for the entire cohort of patients grouped per disease site as well as pictorial and numerical examples of Low risk (A and C) and high risk (B and D) volumes from the hypopharynx/larynx group
Rights and permissions
About this article
Cite this article
Cozzi, L., Franzese, C., Fogliata, A. et al. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther Onkol 195, 805–818 (2019). https://doi.org/10.1007/s00066-019-01483-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01483-0