Abstract
Purpose
The clinical variability of Blakeʼs pouch cysts (BPC) may range from asymptomatic via ataxia to sequelae of decompensated hydrocephalus. On the other hand, Dandy-Walker malformation (DWM) and cerebellar vermis hypoplasia generally correlate with less favorable neurologic development. The aim was to illustrate the potential of prenatal and postnatal neuroimaging to distinguish a BPC or persistent BP from other posterior fossa malformations.
Methods
This pictorial review addresses the inconsistent nomenclature, clinical features, and magnetic resonance imaging (MRI) patterns of BPC and five differential diagnoses. The MRI findings of 11 patients, acquired at up to 3 T in 3 institutions, are demonstrated. Furthermore, the literature was searched for recent improvements in genetic and embryological background knowledge.
Results
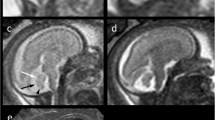
Posterior fossa malformations often resemble each other and may even be imitated by sequelae of hemorrhagic, ischemic or infectious disruptions, i.e. congenital anomalies of morphology despite normal developmental potential. Hydrocephalus is a typical, albeit not always congenital finding in BPC. It is frequently associated with cerebellar disruptions and DWM; however, it is also a rare complication of posterior fossa arachnoid cysts. A moderately elevated vermis needs follow-up to confirm persistent BP versus vermian hypoplasia or DWM. The fetal cerebellar tail, previously assumed to be specific for DWM, may be imitated in cases of persistent BP.
Conclusion
The accurate diagnosis of isolated BPC is not always straightforward, which is especially critical in the context of fetomaternal medicine. A detailed description of posterior fossa malformations is to be preferred over unspecific terminology.











Similar content being viewed by others
References
Gandolfi Colleoni G, Contro E, Carletti A, Ghi T, Campobasso G, Rembouskos G, Volpe G, Pilu G, Volpe P. Prenatal diagnosis and outcome of fetal posterior fossa fluid collections. Ultrasound Obstet Gynecol. 2012;39:625–31.
Doherty D, Millen KJ, Barkovich AJ. Midbrain and hindbrain malformations: advances in clinical diagnosis, imaging, and genetics. Lancet Neurol. 2013;12:381–93.
Tortori-Donati P, Fondelli MP, Rossi A, Carini S. Cystic malformations of the posterior cranial fossa originating from a defect of the posterior membranous area, mega cisterna magna and persisting Blake’s pouch: two separate entities. Childs Nerv Syst. 1996;12:303–8.
Blake JA. The roof and lateral recesses of the fourth ventricle, considered morphologically and embryologically. J Comp Neurol. 1900;10:79–108.
Yildiz H, Yazici Z, Hakyemez B, Erdogan C, Parlak M. Evaluation of CSF flow patterns of posterior fossa cystic malformations using CSF flow MR imaging. Neuroradiology. 2006;48:595–605.
Mohammad SA, Osman NM, Ahmed KA. The value of CSF flow studies in the management of CSF disorders in children: a pictorial review. Insights Imaging. 2019;10:3.
Paladini D, Donarini G, Parodi S, Volpe G, Sglavo G, Fulcheri E. Hindbrain morphometry and choroid plexus position in differential diagnosis of posterior fossa cystic malformations. Ultrasound Obstet Gynecol. 2019;54:207–14.
Huisman TAGM, Poretti A. Disorders of brain development. In: Atlas SW, editor. Magnetic resonance imaging of the brain and spine. 5th ed. Philadelphia: Wolters Kluver; 2017. pp. 116–22.
Nelson MD Jr, Maher K, Gilles FH. A different approach to cysts of the posterior fossa. Pediatr Radiol. 2004;34:720–32.
Robinson AJ. Inferior vermian hypoplasia—preconception, misconception. Ultrasound Obstet Gynecol. 2014;43:123–36.
Calabrò F, Arcuri T, Jinkins JR. Blake’s pouch cyst: an entity within the Dandy-Walker continuum. Neuroradiology. 2000;42:290–5.
Kau T, Birnbacher R, Schwärzler P, Habernig S, Deutschmann H, Boltshauser E. Delayed fenestration of Blake’s pouch with or without vermian hypoplasia: fetal MRI at 3 tesla versus 1.5 tesla. Cerebellum Ataxias. 2019;6:4.
Pinto J, Paladini D, Severino M, Morana G, Pais R, Martinetti C, Rossi A. Delayed rotation of the cerebellar vermis: a pitfall in early second-trimester fetal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2016;48:121–4.
Bontognali M, Poretti A, Guzman R, Huisman TA, Ramelli GP. Blake’s pouch cyst in children: Atypical clinical presentation. Neuroradiol J. 2018;31:430–3.
Cornips EM, Overvliet GM, Weber JW, Postma AA, Hoeberigs CM, Baldewijns MM, Vles JS. The clinical spectrum of Blake’s pouch cyst: report of six illustrative cases. Childs Nerv Syst. 2010;26:1057–64.
Hirono S1, Ito D, Murai H, Kobayashi M, Suyama M, Fujii K, Saeki N. Postnatal development of Blake’s pouch cyst: a case report and new insight for ist pathogenesis. Childs Nerv Syst. 2014;30:1767–71.
Brusius CV, Cavalheiro S. Endoscopic third ventriculostomy is a safe and effective procedure for the treatment of Blake’s pouch cyst. Arq Neuropsiquiatr. 2013;71:545–8.
Boltshauser E, Martin F, Altermatt S. Outcome in children with space-occupying posterior fossa arachnoid cysts. Neuropediatrics. 2002;33:118–21.
De Keersmaecker B, Ramaekers P, Claus F, Witters I, Ortibus E, Naulaers G, Van Calenbergh F, De Catte L. Outcome of 12 antenatally diagnosed fetal arachnoid cysts: case series and review of the literature. Eur J Paediatr Neurol. 2015;19:114–21.
Catala M. The cerebellum and its wrapping meninge: developmental interplay between two major structures. Neuropediatrics. 2017;48:329–39.
Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, Millen KJ. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009;41:1037–42.
Parrini E, Ramazzotti A, Dobyns WB, Mei D, Moro F, Veggiotti P, Marini C, Brilstra EH, Dalla Bernardina B, Goodwin L, Bodell A, Jones MC, Nangeroni M, Palmeri S, Said E, Sander JW, Striano P, Takahashi Y, Van Maldergem L, Leonardi G, Wright M, Walsh CA, Guerrini R. Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain. 2006;129:1892–906.
Lange M, Kasper B, Bohring A, Rutsch F, Kluger G, Hoffjan S, Spranger S, Behnecke A, Ferbert A, Hahn A, Oehl-Jaschkowitz B, Graul-Neumann L, Diepold K, Schreyer I, Bernhard MK, Mueller F, Siebers-Renelt U, Beleza-Meireles A, Uyanik G, Janssens S, Boltshauser E, Winkler J, Schuierer G, Hehr U. 47 patients with FLNA associated periventricular nodular heterotopia. Orphanet J Rare Dis. 2015;10:134.
Poretti A, Boltshauser E. Terminology in morphological anomalies of the cerebellum does matter. Cerebellum ataxias. 2015;2:8.
Ber R, Bar-Yosef O, Hoffmann C, Shashar D, Achiron R, Katorza E. Normal fetal posterior fossa in MR imaging: new biometric data and possible clinical significance. AJNR Am J Neuroradiol. 2015;36:795–802.
Katorza E, Bertucci E, Perlman S, Taschini S, Ber R, Gilboa Y, Mazza V, Achiron R. Development of the fetal vermis: new biometry reference data and comparison of 3 diagnostic modalities-3D ultrasound, 2D ultrasound, and MR imaging. AJNR Am J Neuroradiol. 2016;37:1359–66.
Jandeaux C, Kuchcinski G, Ternynck C, Riquet A, Leclerc X, Pruvo JP, Soto-Ares G. Biometry of the cerebellar vermis and brain stem in children: MR imaging reference data from measurements in 718 children. AJNR Am J Neuroradiol. 2019;40:1835–41.
Poretti A, Boltshauser E, Huisman TAGM. Pre- and postnatal neuroimaging of congenital cerebellar abnormalities. Cerebellum. 2016;15:5–9.
Siegel DH. PHACE syndrome: Infantile hemangiomas associated with multiple congenital anomalies: clues to the cause. Am J Med Genet C Semin Med Genet. 2018;178:407–13.
Poretti A, Boltshauser E, Doherty D. Cerebellar hypoplasia: differential diagnosis and diagnostic approach. Am J Med Genet C Semin Med Genet. 2014;166C:211–26.
Leibovitz Z, Guibaud L, Garel C, Massoud M, Karl K, Malinger G, Haratz KK, Gindes L, Tamarkin M, Ben-Sira L, Lev D, Shalev J, Brasseur-Daudruy M, Contreras Gutierrez de Piñeres CA, Lerman-Sagie T. The cerebellar “tilted telephone receiver sign” enables prenatal diagnosis of PHACES syndrome. Eur J Paediatr Neurol. 2018;22:900–9.
Poretti A, Boltshauser E, Valente EM. The molar tooth sign is pathognomonic for Joubert syndrome! Pediatr Neurol. 2014;50:e15–e6.
Poretti A, Snow J, Summers AC, Tekes A, Huisman TAGM, Aygun N, Carson KA, Doherty D, Parisi MA, Toro C, Yildirimli D, Vemulapalli M, Mullikin JC; NISC Comparative Sequencing Program, Cullinane AR, Vilboux T, Gahl WA, Gunay-Aygun M. Joubert syndrome: neuroimaging findings in 110 patients in correlation with cognitive function and genetic cause. J Med Genet. 2017;54:521–9.
Romaniello R, Arrigoni F, Panzeri E, Poretti A, Micalizzi A, Citterio A, Bedeschi MF, Berardinelli A, Cusmai R, D’Arrigo S, Ferraris A, Hackenberg A, Kuechler A, Mancardi M, Nuovo S, Oehl-Jaschkowitz B, Rossi A, Signorini S, Tüttelmann F, Wahl D, Hehr U, Boltshauser E, Bassi MT, Valente EM, Borgatti R. Tubulin-related cerebellar dysplasia: definition of a distinct pattern of cerebellar malformation. Eur Radiol. 2017;27:5080–92.
D’Antonio F, Khalil A, Garel C, Pilu G, Rizzo G, Lerman-Sagie T, Bhide A, Thilaganathan B, Manzoli L, Papageorghiou AT. Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal ultrasound imaging (part 1): nomenclature, diagnostic accuracy and associated anomalies. Ultrasound Obstet Gynecol. 2016;47:690–7.
Limperopoulos C, Robertson RL, Estroff JA, Barnewolt C, Levine D, Bassan H, du Plessis AJ. Diagnosis of inferior vermian hypoplasia by fetal magnetic resonance imaging: potential pitfalls and neurodevelopmental outcome. Am J Obstet Gynecol. 2006;194:1070–6.
Dandy WE, Blackfan KD. Internal hydrocephalus: an experimental, clinical and pathological study. Am J Dis Child. 1914;8:406–82.
Robinson AJ. Posterior fossa anomalies. In: Kline-Faith BM, Bulas DI, Bahado-Singh R, editors. Fetal imaging—ultrasound and MRI. Philadelphia: Wolters Kluwer; 2015. pp. 430–53.
Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain-hindbrain malformations. Brain. 2009;132:3199–230.
Haldipur P, Dang D, Aldinger KA, Janson OK, Guimiot F, Adle-Biasette H, Dobyns WB, Siebert JR, Russo R, Millen KJ. Phenotypic outcomes in mouse and human Foxc1 dependent dandy-walker cerebellar malformation suggest shared mechanisms. Elife. 2017 Jan 16;6. https://doi.org/10.7554/eLife.20898.
Aldinger KA, Timms AE, Thomson Z, Mirzaa GM, Bennett JT, Rosenberg AB, Roco CM, Hirano M, Abidi F, Haldipur P, Cheng CV, Collins S, Park K, Zeiger J, Overmann LM, Alkuraya FS, Biesecker LG, Braddock SR, Cathey S, Cho MT, Chung BHY, Everman DB, Zarate YA, Jones JR, Schwartz CE, Goldstein A, Hopkin RJ, Krantz ID, Ladda RL, Leppig KA, McGillivray BC, Sell S, Wusik K, Gleeson JG, Nickerson DA, Bamshad MJ, Gerrelli D, Lisgo SN, Seelig G, Ishak GE, Barkovich AJ, Curry CJ, Glass IA, Millen KJ, Doherty D, Dobyns WB. Redefining the etiologic landscape of cerebellar malformations. Am J Hum Genet. 2019;105:606–15.
Bernardo S, Vinci V, Saldari M, Servadei F, Silvestri E, Giancotti A, Aliberti C, Porpora MG, Triulzi F, Rizzo G, Catalano C, Manganaro L. Dandy-Walker malformation: is the ‘tail sign’ the key sign? Prenat Diagn. 2015;35:1358–64.
Brodal A, Hauglie-Hanssen E. Congenital hydrocephalus with defective development of the cerebellar vermis (Dandy-Walker syndrome) clinical and anatomical findings in two cases with particular reference to the so-called atresia of the foramina of Magendie and Luschka. J Neurol Neurosurg Psychiatry. 1959;22:99–108.
Volpe P, Contro E, De Musso F, Ghi T, Farina A, Tempesta A, Volpe G, Rizzo N, Pilu G. Brainstem-vermis and brainstem-tentorium angles allow accurate categorization of fetal upward rotation of cerebellar vermis. Ultrasound Obstet Gynecol. 2012;39:632–5.
Kollias SS, Ball WS Jr, Prenger EC. Cystic malformations of the posterior fossa: differential diagnosis clarified through embryologic analysis. Radiographics. 1993;13:121–31.
Wüest A, Surbek D, Wiest R, Weisstanner C, Bonel H, Steinlin M, Raio L, Tutschek B. Enlarged posterior fossa on prenatal imaging: differential diagnosis, associated anomalies and postnatal outcome. Acta Obstet Gynecol Scand. 2017;96:837–43.
Boddaert N, Klein O, Ferguson N, Sonigo P, Parisot D, Hertz-Pannier L, Baraton J, Emond S, Simon I, Chigot V, Schmit P, Pierre-Kahn A, Brunelle F. Intellectual prognosis of the Dandy-Walker malformation in children: the importance of vermian lobulation. Neuroradiology. 2003;45:320–4.
Martino F, Malova M, Cesaretti C, Parazzini C, Doneda C, Ramenghi LA, Rossi A, Righini A. Prenatal MR imaging features of isolated cerebellar haemorrhagic lesions. Eur Radiol. 2016;26:2685–96.
Messerschmidt A, Brugger PC, Boltshauser E, Zoder G, Sterniste W, Birnbacher R, Prayer D. Disruption of cerebellar development: potential complication of extreme prematurity. AJNR Am J Neuroradiol. 2005;26:1659–67.
Limperopoulos C, Folkerth R, Barnewolt CE, Connolly S, Du Plessis AJ. Posthemorrhagic cerebellar disruption mimicking Dandy-Walker malformation: fetal imaging and neuropathology findings. Semin Pediatr Neurol. 2010;17:75–81.
Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–104.
Bosemani T, Orman G, Boltshauser E, Tekes A, Huisman TA, Poretti A. Congenital abnormalities of the posterior fossa. Radiographics. 2015;35:200–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Kau, R. Marterer, R. Kottke, R. Birnbacher, J. Gellen, E. Nagy and E. Boltshauser declare that they have no competing interests.
Ethical standards
All investigations described in this manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current revised form). Informed consent was obtained from patients if identifiable from images or other information within the manuscript. In the case of underage patients informed consent was obtained from the legal representatives.
Additional information
Author Contributions
T. Kau had the idea for this article, performed literature search, provided data, and drafted the article, E. Boltshauser performed literature search, provided data, and critically revised the article, R. Kottke provided data and critically revised the article, R. Marterer, R. Birnbacher, J. Gellen and E. Nagy commented on the data and critically revised the manuscript.
Rights and permissions
About this article
Cite this article
Kau, T., Marterer, R., Kottke, R. et al. Blakeʼs Pouch Cysts and Differential Diagnoses in Prenatal and Postnatal MRI. Clin Neuroradiol 30, 435–445 (2020). https://doi.org/10.1007/s00062-019-00871-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00871-4