Abstract
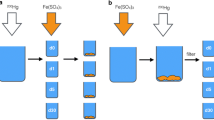
The efficient removal of dissolved organic matter (DOM) from drinking water is important to protect public health. However, the changes to DOM during drinking water treatments are poorly understood on a molecular level. Here, we investigated the changes induced by permanganate oxidation, alum coagulation, and activated carbon sorption for three DOM isolates, one derived from terrestrial materials, Suwanee River fulvic acid (SRFA), and two derived from microbial inputs, Grand Lake St. Mary’s (GLSM) DOM, and Jackson Pike wastewater effluent DOM (EfOM). These treatments were investigated individually, and in realistic treatment sequences, using conventional bulk characterization techniques and ultrahigh-resolution mass spectrometry. We found that permanganate pre-oxidation improved DOM removal by subsequent alum coagulation through the oxidation of aromatic components and the formation of tannin-like components. This effect was most prominent for SRFA and was less pronounced for the microbial DOM isolates. The impact of permanganate pre-oxidation had little to no effect on subsequent activated carbon treatment following coagulation. Removal of SRFA was impacted by the application order of alum coagulation and activated carbon sorption with coagulation followed by activated carbon sorption leading to the greatest organic matter removal efficiency. The favorable efficacy of this treatment series is likely caused by the removal of high molecular weight organic matter components by coagulation that would otherwise block sorption to activated carbon sites. Treatment application order impacted the removal of GLSM DOM and EfOM to a lesser extent, resulting in similar overall organic matter removal efficiencies that could be treated as additive for coagulation and activated carbon sorption. These observations, overall, suggest that permanganate pre-oxidation is most effective for improving DOM removal when aromatic terrestrial inputs are present, and pretreatments, including permanganate oxidation and activated carbon sorption, may be most effective when microbial inputs are present due to the absence of aromatic chemical species.





Similar content being viewed by others
Data availability
The FT-ICR-MS and UV–Vis spectroscopy datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request. Bulk parameter datasets are available in the Supporting Information file.
References
Aung MT, Shimabuku K, Soares-Quinete N, Kearns JP (2022) Leveraging DOM UV absorbance and fluorescence to accurately predict and monitor short-chain PFAS removal by fixed-bed carbon adsorbers. Water Res 213:11
Bahureksa W, Tfaily MM, Boiteau RM, Young RB, Logan MN, McKenna AM, Borch T (2021) Soil organic matter characterization by fourier transform ion cyclotron resonance mass spectrometry (FTICR MS): a critical review of sample preparation, analysis, and data interpretation. Environ Sci Technol. https://doi.org/10.1021/acs.est.1c01135
Bajracharya A, Liu Y-L, Lenhart JJ (2019) The influence of natural organic matter on the adsorption of microcystin-LR by powdered activated carbon. Environ Sci: Water Res Technol 5(2):256–267
Ballard BD, MacKay AA (2005) Estimating the removal of anthropogenic organic chemicals from raw drinking water by coagulation flocculation. J Environ Eng 131(1):108–118
Benjamin MM, Lawler DF (2013) Water quality engineering: physical/chemical treatment processes. Wiley, Hoboken
Blackburn JWT, Kew W, Graham MC, Uhrin D (2017) Laser desorption/ionization coupled to FTICR mass spectrometry for studies of natural organic matter. Anal Chem 89(8):4382–4386
Brown A, McKnight DM, Chin Y-P, Roberts EC, Uhle M (2004) Chemical characterization of dissolved organic material in Pony Lake, a saline coastal pond in Antarctica. Mar Chem 89(1–4):327–337
Cao D, Huang H, Hu M, Cui L, Geng F, Rao Z, Niu H, Cai Y, Kang Y (2015) Comprehensive characterization of natural organic matter by MALDI- and ESI-Fourier transform ion cyclotron resonance mass spectrometry. Anal Chim Acta 866:48–58
Chowdhury FL, Bérubé PR, Mohseni M (2008) Characteristics of natural organic matter and formation of chlorinated disinfection by-products from two source waters that respond differently to ozonation. Ozone Sci Eng 30(5):321–331
Colthurst JM, Singer PC (1982) Removing trihalomethane precursors by permanganate oxidation and manganese dioxide adsorption. J Am Water Works Assoc 74(2):78–83
Cui H, Huang X, Yu Z, Chen P, Cao X (2020) Application progress of enhanced coagulation in water treatment. RSC Adv 10(34):20231–20244
Cuthbertson AA, Kimura SY, Liberatore HK, Summers RS, Knappe DRU, Stanford BD, Maness JC, Mulhern RE, Selbes M, Richardson SD (2019) Does granular activated carbon with chlorination produce safer drinking water? From disinfection byproducts and total organic halogen to calculated toxicity. Environ Sci Technol 53(10):5987–5999
Dash S, Patel S, Mishra BK (2009) Oxidation by permanganate: synthetic and mechanistic aspects. TET Tetrahedron 65(4):707–739
Dittmar TKB, Hertkorn N, Kattner G (2008) A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol Oceanogr Methods 6:230–235
Dotson A, Westerhoff P (2009) Occurrence and removal of amino acids during drinking water treatment. J Am Water Works Assoc 101(9):101–115
Dunn SE, Knappe DR (2013) DBP precursor micropollutant removal by powdered activated carbon. Water Research Foundation, Denver
Edwards M (1997) Predicting DOC removal during enhanced coagulation. J Am Water Works Assoc 89(5):78–89
Gonsior M, Schmitt-Kopplin P, Stavklint H, Richardson SD, Hertkorn N, Bastviken D (2014) Changes in dissolved organic matter during the treatment processes of a drinking water plant in Sweden and formation of previously unknown disinfection byproducts. Environ Sci Technol 48(21):12714–12722
Gross JH (2011) Mass spectrometry: a textbook. Springer, Meppel
Guan X, He D, Ma J, Chen G (2010) Application of permanganate in the oxidation of micropollutants: a mini review. Front Environ Sci Eng China 4(4):405–413
Jaky M, Simandi LI (1976) Mechanism of the permanganate oxidation of unsaturated compounds. Part VI. kinetic investigation of the oxidation of methylmaleic acid, methylfumaric acid, and dimethylmaleic acid. J Chem Soc, Perkin Trans 2:939–943
Jiang J, Gao Y, Pang SY, Wang Q, Huangfu X, Liu Y, Ma J (2014) Oxidation of bromophenols and formation of brominated polymeric products of concern during water treatment with potassium permanganate. Environ Sci Technol 48(18):10850–10858
Kennedy AM, Summers RS (2015) Effect of DOM size on organic micropollutant adsorption by GAC. Environ Sci Technol 49(11):6617–6624
Kew W, Mackay CL, Goodall I, Clarke DJ, Uhrin D (2018) Complementary ionization techniques for the analysis of scotch whisky by high resolution mass spectrometry. Anal Chem 90(19):11265–11272
Koch BP, Dittmar T (2006) From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun Mass Spectrom 20(5):926–932
Koch BP, Dittmar T (2016) From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun Mass Spectrom 30(1):250–250
Korak JA, Rosario-Ortiz FL, Scott Summers R (2015) Evaluation of optical surrogates for the characterization of DOM removal by coagulation. Environ Sci: Water Res Technol 1(4):493–506
Kruve A, Kaupmees K (2017) Adduct formation in ESI/MS by mobile phase additives. J Am Soc Mass Spectrom 28(5):887–894
Kruve A, Kaupmees K, Liigand J, Oss M, Leito I (2013) Sodium adduct formation efficiency in ESI source. J Mass Spectrom 48(6):695–702
Laszakovits JR, MacKay AA (2021) Data-based chemical class regions for Van Krevelen diagrams. J Am Soc Mass Spectrom 33(1):198–202
Laszakovits JR, Somogyi A, MacKay AA (2020) Chemical alterations of dissolved organic matter by permanganate oxidation. Environ Sci Technol. https://doi.org/10.1021/acs.est.9b06675
Laszakovits JR, Kerr A, MacKay AA (2022) Permanganate oxidation of organic contaminants and model compounds. Environ Sci Technol 56(8):4728–4748
Lavonen EE, Gonsior M, Tranvik LJ, Schmitt-Kopplin P, Kohler SJ (2013) Selective chlorination of natural organic matter: identification of previously unknown disinfection byproducts. Environ Sci Technol 47(5):2264–2271
Leveque B, Burnet JB, Dorner S, Bichai F (2021) Impact of climate change on the vulnerability of drinking water intakes in a northern region. Sustain Cities Soc. https://doi.org/10.1016/j.scs.2020.102656
Li XF, Mitch WA (2018) Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52(4):1681–1689
Ma L, Peng F, Lu Y, Yang Z, Qiu B, Li H (2022) The effect of coagulation on the removal of algogenic organic matter and the optical parameters for predicting disinfection byproducts. Sep Purif Technol 280:119906. https://doi.org/10.1016/j.seppur.2021.119906
Maizel AC, Remucal CK (2017) The effect of advanced secondary municipal wastewater treatment on the molecular composition of dissolved organic matter. Water Res 122:42–52
Marsh H, Rodriguez-Reinoso F (2006) Activated carbon. Elsevier, Oxford
Milstead RP, Remucal CK (2021) Molecular-level insights into the formation of traditional and novel halogenated disinfection byproducts. ACS EST Water. https://doi.org/10.1021/acsestwater.1c00161
Mugo SM, Bottaro CS (2004) Characterization of humic substances by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 18(20):2375–2382
Naceradska J, Pivokonsky M, Pivokonska L, Baresova M, Henderson RK, Zamyadi A, Janda V (2017) The impact of pre-oxidation with potassium permanganate on cyanobacterial organic matter removal by coagulation. Water Res 114:42–49
Nam SN, Amy G (2008) Differentiation of wastewater effluent organic matter (EfOM) from natural organic matter (NOM) using multiple analytical techniques. Water Sci Technol 57(7):1009–1015
Ohno T, Sleighter RL, Hatcher PG (2016) Comparative study of organic matter chemical characterization using negative and positive mode electrospray ionization ultrahigh-resolution mass spectrometry. Anal Bioanal Chem 408(10):2497–2504
Pei J, Hsu C-C, Wang Y, Yu K (2017) Corona discharge-induced reduction of quinones in negative electrospray ionization mass spectrometry. RSC Adv 7(69):43540–43545
Peng S, Zhang X, Zhao X, Zhang Y (2015) A review of different drinking water treatments for natural organic matter removal. Water Supply 15(3):442–455
Phungsai P, Kurisu F, Kasuga I, Furumai H (2018) Changes in dissolved organic matter composition and disinfection byproduct precursors in advanced drinking water treatment processes. Environ Sci Technol 52(6):3392–3401
Piezer K, Li L, Jeon Y, Kadudula A, Seo Y (2021) The Application of potassium permanganate to treat cyanobacteria-laden water: a review. Process Saf Environ Prot 148:400–414
Rudakov ES, Lobachev VL (2000) The first step of oxidation of alkylbenzenes by permanganates in acidic aqueous solutions. Russ Chem Bull 49(5):761–777
Shi W, Zhuang WE, Hur J, Yang L (2021) Monitoring dissolved organic matter in wastewater and drinking water treatments using spectroscopic analysis and ultra-high resolution mass spectrometry. Water Res 188:116406
Shimabuku KK, Cho H, Townsend EB, Rosario-Ortiz FL, Summers RS (2014) Modeling nonequilibrium adsorption of MIB and sulfamethoxazole by powdered activated carbon and the role of dissolved organic matter competition. Environ Sci Technol 48(23):13735–13742
Shimabuku KK, Paige JM, Luna-Aguero M, Summers RS (2017) Simplified modeling of organic contaminant adsorption by activated carbon and biochar in the presence of dissolved organic matter and other competing adsorbates. Environ Sci Technol 51(17):10031–10040
Sillanpaa M, Ncibi MC, Matilainen A, Vepsalainen M (2018) Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere 190:54–71
Simandi LI, Jaky M, Son NT, Hegedus-Vajda J (1977) Mechanism of the permanganate oxidation of unsaturated compounds. Part 8. Kinetics of the oxidation of halogenomaleic acids. J Chem Soc Perkin Trans 2:1794–1798
Singer PC, Borchardt JH, Colthurst JM (1980) The effects of permanganate pretreatment on trihalomethane formation in drinking water. J Am Water Works Assoc 72(10):573–578
Steffen MM, Zhu Z, McKay RML, Wilhelm SW, Bullerjahn GS (2014) Taxonomic assessment of a toxic cyanobacteria shift in hypereutrophic Grand Lake St. Marys (Ohio, USA). Harmful Algae 33:12–18
Summers RS, Shiokari ST, Johnson S, Pereterson E, Yu Y, Cook S (2020) Reuse treatment with ozonation, biofiltration, and activated carbon adsorption for total organic carbon control and disinfection byproduct regulation compliance. AWWA Water Sci. https://doi.org/10.1002/aws2.1190
Velten S, Knappe DR, Traber J, Kaiser HP, von Gunten U, Boller M, Meylan S (2011) Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res 45(13):3951–3959
von Gunten U (2018) Oxidation processes in water treatment: are we on track? Environ Sci Technol 52(9):5062–5075
Waldemer RH, Tratnyek PG (2006) Kinetics of contaminant degradation by permanganate. Environ Sci Technol 40(3):1055–1061
Wang D, Xie J, Chow CWK, Xing L, van Leeuwen J (2012) Characterization and predicting DOM treatability by enhanced coagulation. Water Sci Technol Water Supply 13(1):147
Wang D, Zhao Y, Xie J, Chow CWK, van Leeuwen J (2013) Characterizing DOM and removal by enhanced coagulation: a survey with typical Chinese source waters. Sep Purif Technol 110:188–195
Wang F, Gao B, Yue Q, Bu F, Shen X (2017) Effects of ozonation, powdered activated carbon adsorption, and coagulation on the removal of disinfection by-product precursors in reservoir water. Environ Sci Pollut Res 24(21):17945–17954
Ward CP, Cory RM (2015) Chemical composition of dissolved organic matter draining permafrost soils. Geochim Cosmochim Acta 167:63–79
Watson K, Farre MJ, Leusch FDL, Knight N (2018) Using fluorescence-parallel factor analysis for assessing disinfection by-product formation and natural organic matter removal efficiency in secondary treated synthetic drinking waters. Sci Total Environ 640–641:31–40
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorption as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Westerhoff P, Mash H (2002) Dissolved organic nitrogen in drinking water supplies: a review. J Water Supply Res Technol AQUA 51(8):415–448
Xie P, Ma J, Fang J, Guan Y, Yue S, Li X, Chen L (2013) Comparison of permanganate preoxidation and preozonation on algae containing water: cell integrity, characteristics, and chlorinated disinfection byproduct formation. Environ Sci Technol 47(24):14051–14061
Zietzschmann F, Worch E, Altmann J, Ruhl AS, Sperlich A, Meinel F, Jekel M (2014) Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res 65:297–306
Acknowledgements
This work was supported through a Presidential Fellowship awarded by The Ohio State University Graduate School and a grant from the Ohio Water Development Authority. We thank the Mass Spectrometry and Proteomics CCIC at The Ohio State University for allowing us to use the Bruker SolariX ICR-MS. The 15 T Bruker SolariXR FT-ICR instrument was supported by NIH Award Number Grant S10 OD018507. The project was supported by NIH Award Number Grant P30 CA016058. We are especially thankful for the support and guidance of Dr. Arpad Somogyi, the director of the MS CCIC. We also thank the Jackson Pike Wastewater treatment plant staff who assisted us with sampling water from their treatment plant. We would also like to acknowledge the input of treatment plant operators in Ohio, particularly, Todd Hone formerly at the Celina drinking water treatment plant for helping us to understand the impact of algal blooms on drinking water treatment plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laszakovits, J.R., MacKay, A.A. The impact of permanganate pre-oxidation on subsequent drinking water treatment operations. Aquat Sci 85, 33 (2023). https://doi.org/10.1007/s00027-022-00916-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00916-w