Abstract
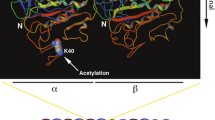
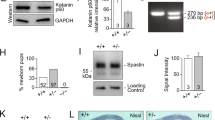
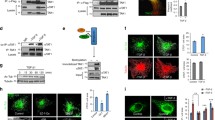
α-Tubulin acetyltransferase 1 (ATAT1) catalyzes acetylation of α-tubulin at lysine 40 in various organisms ranging from Tetrahymena to humans. Despite the importance in mammals suggested by studies of cultured cells, the mouse Atat1 gene is non-essential for survival, raising an intriguing question about its real functions in vivo. To address this question, we systematically analyzed a mouse strain lacking the gene. The analyses revealed that starting at postnatal day 5, the mutant mice display enlarged lateral ventricles in the forebrain, resembling ventricular dilation in human patients with ventriculomegaly. In the mice, ventricular dilation is due to hypoplasia in the septum and striatum. Behavioral tests of the mice uncovered deficits in motor coordination. Birth-dating experiments revealed that neuronal migration to the mutant septum and striatum is impaired during brain development. In the mutant embryonic fibroblasts, we found mild defects in cell proliferation and primary cilium formation. Notably, in these cells, ATAT1 is indispensable for tubulin hyperacetylation in response to high salt, high glucose, and hydrogen peroxide-induced oxidative stress. We investigated the role of ATAT1 in the hematopoietic system using multicolor flow cytometry and found that this system remains normal in the mutant mice. Although tubulin acetylation was undetectable in a majority of mutant tissues, residual levels were detected in the heart, skeletal muscle, trachea, oviduct, thymus and spleen. This study thus not only establishes the importance of ATAT1 in regulating mouse forebrain development and governing tubulin hyperacetylation during stress responses, but also suggests the existence of an additional α-tubulin acetyltransferase.









Similar content being viewed by others
References
Lhernault SW, Rosenbaum JL (1985) Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24:473–478
Ledizet M, Piperno G (1987) Identification of an acetylation site of chlamydomonas alpha-tubulin. Proc Natl Acad Sci USA 84:5720–5724
Piperno G, Ledizet M, Chang XJ (1987) Microtubules containing acetylated alpha-tubulin in mammalian-cells in culture. J Cell Biol 104:289–302
Piperno G, Fuller MT (1985) Monoclonal-antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101:2085–2094
Nakagawa U, Suzuki D, Ishikawa M, Sato H, Kamemura K, Imamura A (2013) Acetylation of alpha-Tubulin on Lys(40) is a widespread post-translational modification in angiosperms. Biosci Biotech Bioch 77:1602–1605
Yang XJ, Grégoire S (2005) Class II histone deacetylases: from sequence to function, regulation and clinical implication. Mol Cell Biol 25:2873–2884
Topalidou I, Keller C, Kalebic N, Nguyen KCQ, Somhegyi H, Politi KA, Heppenstall P, Hall DH, Chalfie M (2012) Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol 22:1057–1065
Cueva JG, Hsin J, Huang KC, Goodman MB (2012) Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr Biol 22:1066–1074
Reed NA, Cai DW, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172
Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F (2007) Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci 27:3571–3583
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458
Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22:1168–1179
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831
Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P (2008) Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28:1688–1701
Janke C, Montagnac G (2017) Causes and consequences of microtubule acetylation. Curr Biol 27:R1287–R1292
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J (2010) MEC-17 is an alpha-tubulin acetyltransferase. Nature 467:218–222
Shida T, Cueva JG, Xu ZJ, Goodman MB, Nachury MV (2010) The major alpha-tubulin K40 acetyltransferase alpha TAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA 107:21517–21522
Li L, Wei D, Wang Q, Pan J, Liu R, Zhang X, Bao L (2012) MEC-17 deficiency leads to reduced alpha-tubulin acetylation and impaired migration of cortical neurons. J Neurosci 32:12673–12683
Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, Goloubeva OG, Ioffe OB, Tuttle KC, Slovic J, Lu YL, Mills GB, Martin SS (2015) alpha-tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res 75:203–215
Li L, Yang XJ (2015) Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci 72:4237–4255
Kim GW, Li L, Ghorbani M, You L, Yang XJ (2013) Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. J Biol Chem 288:20334–20350
Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA (2013) alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun 4:1962
Morley SJ, Qi YM, Iovino L, Andolfi L, Guo D, Kalebic N, Castaldi L, Tischer C, Portulano C, Bolasco G, Shirlekar K, Fusco CM, Asaro A, Fermani F, Sundukova M, Matti U, Reymond L, De Ninno A, Businaro L, Johnsson K, Lazzarino M, Ries J, Schwab Y, Hu J, Heppenstall PA (2016) Acetylated tubulin is essential for touch sensation in mice. Elife 5
Howes SC, Alushin GM, Shida T, Nachury MV, Nogales E (2014) Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol Biol Cell 25:257–266
Lin X, Liu BH, Yang XS, Yue XJ, Diao LX, Wang J, Chang J (2013) Genetic deletion of Rnd3 results in aqueductal stenosis leading to hydrocephalus through up-regulation of Notch signaling. Proc Natl Acad Sci USA 110:8236–8241
Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK (2005) Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132:5329–5339
Li L, Yang XJ (2015) Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci 72:4237–4255
Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L (2009) Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136:551–564
Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M (2009) Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12:463–473
Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129:1351–1363
Prodromou NV, Thompson CL, Osborn DPS, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP (2012) Heat shock induces rapid resorption of primary cilia. J Cell Sci 125:4297–4305
Xie R, Nguyen S, McKeehan WL, Liu LY (2010) Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 11:89
Banreti A, Sass M, Graba Y (2013) The emerging role of acetylation in the regulation of autophagy. Autophagy 9:819–829
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60:543–554
Ma Q, Yang JM, Li T, Milner TA, Hempstead BL (2015) Selective reduction of striatal mature BDNF without induction of proBDNF in the zQ175 mouse model of Huntington’s disease. Neurobiol Dis 82:466–477
Iwata A, Riley BE, Johnston JA, Kopito RR (2005) HDAC6 and microtubules are required for autophagic degradation of aggregated Huntingtin. J Biol Chem 280:40282–40292
Guedes-Dias P, de Proenca J, Soares TR, Leitao-Rocha A, Pinho BR, Duchen MR, Oliveira JMA (2015) HDAC6 inhibition induces mitochondrial fusion, autophagic flux and reduces diffuse mutant huntingtin in striatal neurons. Bba-Mol Basis Dis 1852:2484–2493
Simoes-Pires C, Zwick V, Nurisso A, Schenker E, Carrupt PA, Cuendet M (2013) HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? Mol Neurodegener 8
Bobrowska A, Paganetti P, Matthias P, Bates GP (2011) Hdac6 knock-out increases tubulin acetylation but does not modify disease progression in the R6/2 mouse model of huntington’s disease. PLoS ONE 6:e20696
Shah N, Kumar S, Zaman N, Pan CC, Bloodworth JC, Lei W, Streicher JM, Hempel N, Mythreye K, Lee NY (2018) TAK1 activation of alpha-TAT1 and microtubule hyperacetylation control AKT signaling and cell growth. Nat Commun 9:1696
Oh S, You E, Ko P, Jeong J, Keum S, Rhee S (2017) Genetic disruption of tubulin acetyltransferase, alpha TAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/beta-catenin signaling. Biochem Bioph Res Co 482:8–14
Geeraert C, Ratier A, Pfisterer SG, Perdiz D, Cantaloube I, Rouault A, Pattingre S, Proikas-Cezanne T, Codogno P, Pous C (2010) Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem 285:24184–24194
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Fratzer C, Krannich A, Gollasch M, Grohme DA, Corte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Muller DN (2017) Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551:585–589
Walkinshaw DR, Weist R, Kim GW, You L, Xiao L, Nie J, Li CS, Zhao S, Xu M, Yang XJ (2013) The tumor suppressor kinase LKB1 activates the downstream kinases SIK2 and SIK3 to stimulate nuclear export of class IIa histone deacetylases. J Biol Chem 288:9345–9362
Wang B, Rao YH, Inoue M, Hao R, Lai CH, Chen D, McDonald SL, Choi MC, Wang Q, Shinohara ML, Yao TP (2014) Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun 5:3479
Wong VSC, Picci C, Swift M, Levinson M, Willis D, Langley B (2018) alpha-Tubulin acetyltransferase is a novel target mediating neurite growth inhibitory effects of chondroitin sulfate proteoglycans and myelin-associated glycoprotein. eNeuro. https://doi.org/10.1523/ENEURO.0240-0217.2018
Xu ZJ, Schaedel L, Portran D, Aguilar A, Gaillard J, Marinkovich MP, Thery M, Nachury MV (2017) Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356:328–332
Portran D, Schaedel L, Xu ZJ, Thery M, Nachury MV (2017) Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol 19:391
Solinger JA, Paolinelli R, Kloss H, Scorza FB, Marchesi S, Sauder U, Mitsushima D, Capuani F, Sturzenbaum SR, Cassata G (2010) The Caenorhabditis elegans elongator complex regulates neuronal alpha-tubulin acetylation. Plos Genet 6
Conacci-Sorrell M, Ngouenet C, Eisenman RN (2010) Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation. Cell 142:480–493
Ohkawa N, Sugisaki S, Tokunaga E, Fujitani K, Hayasaka T, Setou M, Inokuchi K (2008) N-acetyltransferase ARD1-NAT1 regulates neuronal dendritic development. Genes Cells 13:1171–1183
Sadoul K, Wang J, Diagouraga B, Vitte AL, Buchou T, Rossini T, Polack B, Xi XD, Matthias P, Khochbin S (2012) HDAC6 controls the kinetics of platelet activation. Blood 120:4215–4218
Messaoudi K, Ali A, Ishaq R, Palazzo A, Sliwa D, Bluteau O, Souquere S, Muller D, Diop KM, Rameau P, Lapierre V, Marolleau JP, Matthias P, Godin I, Pierron G, Thomas SG, Watson SP, Droin N, Vainchenker W, Plo I, Raslova H, Debili N (2017) Critical role of the HDAC6-cortactin axis in human megakaryocyte maturation leading to a proplatelet-formation defect. Nat Commun 8:1786
Wang N, Tall AR (2016) Cholesterol in platelet biogenesis and activation. Blood 127:1949–1953
Krishnegowda M, Rajashekaraiah V (2015) Platelet disorders: an overview. Blood Coagul Fibrinolysis 26:479–491
Sobreira N, Schiettecatte F, Valle D, Hamosh A (2015) GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36:928–930
Yan K, Rousseau J, Littlejohn RO, Kiss C, Lehman A, Rosenfeld JR, Stumpel CTR, Stegmann AP, Robak L, Scaglia F, Nguyen TT, Fu H, Ajeawung NF, Camurri MV, Li L, Gardham A, Panis B, Almannai M, Sacoto MJ, Baskin B, Ruivenkamp C, Study D, Study C, Cho MT, Potjer T, Santen GW, Parker MJ, Canham N, McKinnon M, Potocki L, MacKenzie J, Roeder ER, Campeau PM, Yang XJ (2017) Mutations in the chromatin regulator gene BRPF1 causes syndromic intellectual disability and deficient histone acetylation. Am J Hum Genet 100:91–104
You L, Yan K, Zou J, Zhao H, Bertos NR, Park M, Wang E, Yang XJ (2015) The lysine acetyltransferase activator Brpf1 governs dentate gyrus development through neural stem cells and progenitors. PLoS Genet 11:e1005034
You L, Zou J, Zhao H, Bertos NR, Park M, Wang E, Yang XJ (2015) Deficiency of the chromatin regulator Brpf1 causes abnormal brain development. J Biol Chem 290:7114–7129
Sansregret L, Vadnais C, Livingstone J, Kwiatkowski N, Awan A, Cadieux C, Leduy L, Hallett MT, Nepveu A (2011) Cut homeobox 1 causes chromosomal instability by promoting bipolar division after cytokinesis failure. Proc Natl Acad Sci USA 108:1949–1954
Gingras H, Cases O, Krasilnikova M, Berube G, Nepveu A (2005) Biochemical characterization of the mammalian Cux2 protein. Gene 344:273–285
You L, Zou J, Zhao H, Bertos NR, Park M, Wang E, Yang XJ (2015) Deficiency of the chromatin regulator BRPF1 causes abnormal brain development. J Biol Chem 290:7114–7129
Heinsbroek RPW, Vanhaaren F, Vandepoll NE (1988) Sex-differences in passive-avoidance behavior of rats—sex-dependent susceptibility to shock-induced behavioral depression. Physiol Behav 43:201–206
Yan K, You L, Degerny C, Ghorbani M, Liu X, Chen L, Li L, Miao D, Yang XJ (2016) The chromatin regulator BRPF3 preferentially activates the HBO1 acetyltransferase but is dispensable for mouse development and survival. J Biol Chem 291:2647–2663
You L, Li L, Yan K, Zou J, Belle J, Nijnik A, Wang E, Yang XJ (2016) BRPF1 is essential for development of fetal hematopoietic stem cells. J Clin Invest 126:3247–3262
Acknowledgements
This work was supported by research grants from Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada and the Canadian Cancer Society (to X.-J.Y.). L.L. received stipend support from the China Scholarship Council, the Clifford C.F. Wong Fellowship program, a CIHR/FRSQ training grant in cancer research for the McGill Integrated Cancer Research Training Program and the Canderel Foundation.
Author information
Authors and Affiliations
Contributions
LL carried out all experiments and wrote the manuscript; SJ and AW supervised the rotarod tests; MM helped perform some experiments with MEFs; LML and SM assisted with open field tests; XJY supervised the project and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest with the contents of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Jayabal, S., Ghorbani, M. et al. ATAT1 regulates forebrain development and stress-induced tubulin hyperacetylation. Cell. Mol. Life Sci. 76, 3621–3640 (2019). https://doi.org/10.1007/s00018-019-03088-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03088-3