Abstract
Background
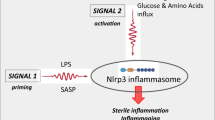
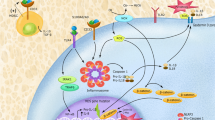
The inflammasome is a cytosolic multi-protein complex responsible for the proteolytic maturation of pro-inflammatory cytokines IL-1ß and IL-18 and of gasdermin-D, which mediates membrane pore formation and the cytokines release, or eventually a lytic cell death known as pyroptosis. Inflammation has long been accepted as a key component of hematologic conditions, either oncological or benign diseases.
Objectives
This study aims to review the current knowledge about the contribution of inflammasome in hematologic diseases. We attempted to depict the participation of specific inflammasome receptors, and the possible cell-specific consequence of complex activation, as well as the use of anti-inflammasome therapies.
Methods
We performed a keyword-based search in public databases (Pubmed.gov, ClinicalTrials.gov.).
Conclusion
Different blood cells variably express inflammasome components. Considering the immunosuppression associated with both the disease and the treatment of some hematologic diseases, and a microenvironment that allows neoplastic cell proliferation, inflammasomes could be a link between innate immunity and disease progression, as well as an interesting therapeutic target.

Similar content being viewed by others
References
Tartey S, Kanneganti TD. Inflammasomes in the pathophysiology of autoinflammatory syndromes. J Leukoc Biol. 2020;107(3):379–91. https://doi.org/10.1002/JLB.3MIR0919-191R.
Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases, and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. https://doi.org/10.1111/imr.12534.
Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17(2):57–64. https://doi.org/10.1016/j.molmed.2010.11.001.
Tong Y, Wang Z, Cai L, Lin L, Liu J, Cheng J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid Med Cell Longev. 2020;14(2020):4293206. https://doi.org/10.1155/2020/4293206.
Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477(6):1089–107. https://doi.org/10.1042/BCJ20190472.
Chung C, Seo W, Silwal P, Jo EK. Crosstalks between inflammasome and autophagy in cancer. J Hematol Oncol. 2020;13(1):100. https://doi.org/10.1186/s13045-020-00936-9.
Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, Cengia LH, Henley KJ, Collinge JE, Kastner DL, Feigenbaum L, Hilton DJ, Alexander WS, Kile BT, Croker BA. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37(6):1009–23. https://doi.org/10.1016/j.immuni.2012.08.027.
Rodríguez-Ruiz L, Lozano-Gil JM, Lachaud C, Mesa-Del-Castillo P, Cayuela ML, García-Moreno D, Pérez-Oliva AB, Mulero V. Zebrafish Models to Study inflammasome-mediated regulation of hematopoiesis. Trends Immunol. 2020;41(12):1116–27. https://doi.org/10.1016/j.it.2020.10.006.
Martinon F, Burns K, Tschopp J. 2002 The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. https://doi.org/10.1016/s1097-2765(02)00599-3.
Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8. https://doi.org/10.1038/nature18629.
Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–52. https://doi.org/10.1016/j.it.2019.09.005.
Sutterwala FS, Flavell RA. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin Immunol. 2009;130(1):2–6. https://doi.org/10.1016/j.clim.2008.08.011.
Lugrin J, Martinon F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol Rev. 2018;281(1):99–114. https://doi.org/10.1111/imr.12618.
Tsu BV, Beierschmitt C, Ryan AP, Agarwal R, Mitchell PS, Daugherty MD. Diverse viral proteases activate the NLRP1 inflammasome. Elife. 2021;10: e60609. https://doi.org/10.7554/eLife.60609.
Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–41. https://doi.org/10.1038/nature13449.
Chen Q, Shi P, Wang Y, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11(6):496–508. https://doi.org/10.1093/jmcb/mjy056.
Ito M, Shichita T, Okada M, et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun. 2015;6:7360. https://doi.org/10.1038/ncomms8360.
Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–46. https://doi.org/10.4049/jimmunol.1302839.
Chui AJ, Griswold AR, Taabazuing CY, et al. Activation of the CARD8 inflammasome requires a disordered region. Cell Rep. 2020;33(2): 108264. https://doi.org/10.1016/j.celrep.2020.108264.
Le HT, Harton JA. Pyrin- and CARD-only proteins as regulators of NLR functions. Front Immunol. 2013;17(4):275. https://doi.org/10.3389/fimmu.2013.00275.
Papatheodorou I, Moreno P, Manning J, Fuentes AMP, George N, Fexova S, Fonseca NA, et al. Expression Atlas update: from tissues to single cells. Nucleic Acids Res. 2020;48(D1):D77–83. https://doi.org/10.1093/nar/gkx1158.
Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascualr and other tissues. Int J Immunopathol Pharmacol. 2009;22(2):311–22. https://doi.org/10.1177/039463200902200208.
Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. https://doi.org/10.1146/annurev-immunol-031210-101405.
Jagannathan-Bogdan M, Zon LI. Hematopoiesis. Development. 2013;140(12):2463–7. https://doi.org/10.1242/dev.083147.
Ratajczak MZ, Adamiak M, Thapa A, Bujko K, Brzezniakiewicz-Janus K, Lenkiewicz AM. NLRP3 inflammasome couples purinergic signaling with activation of the complement cascade for the optimal release of cells from bone marrow. Leukemia. 2019;33(4):815–25. https://doi.org/10.1038/s41375-019-0436-6.
Hong F, Chen Y, Gao H, Shi J, Lu W, Ju W, Fu C, Qiao J, Xu K, Zeng L. NLRP1 in bone marrow microenvironment controls hematopoietic reconstitution after transplantation. Transplant Cell Ther. 2021;S2666–6367(21):01098–108. https://doi.org/10.1016/j.jtct.2021.07.016.
Hu B, Jin CC, Li HB, Tong JY, Ouyang XS, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–8. https://doi.org/10.1126/science.aaf7532.
Yang L, Hu M, Lu Y, Han S, Wang J. Inflammasomes and the maintenance of hematopoietic homeostasis: new perspectives and opportunities. Molecules. 2021;26:309. https://doi.org/10.3390/molecules26020309.
Lenkiewicz AM, Adamiak M, Thapa A, Bujko K, Pedziwiatr D, Abdel-Latif AK, Kucia M, Ratajczak J, Ratajczak MZ. The Nlrp3 inflammasome orchestrates mobilization of bone marrow-residing stem cells into peripheral blood. Stem Cell Rev Rep. 2019;15(3):391–403. https://doi.org/10.1007/s12015-019-09890-7.
Espinoza JL, Kamio K, Lam VQ, Takami A. The impact of NLRP3 activation on hematopoietic stem cell transplantation. Int J Mol Sci. 2021;22(21):11845. https://doi.org/10.3390/ijms222111845.
Bozza MT, Jeney V. Pro-inflammatory Actions of heme and other hemoglobin-derived DAMPs. Front Immunol. 2020;11:1323. https://doi.org/10.3389/fimmu.2020.01323.
Vogel S, Arora T, Wang X, Mendelsohn L, Nichols J, Allen D, Shet AS, Combs CA, Quezado ZMN, Thein SL. The platelet NLRP3 inflammasome is upregulated in sickle cell disease via HMGB1/TLR4 and Bruton tyrosine kinase. Blood Adv. 2018;2(20):2672–80. https://doi.org/10.1182/bloodadvances.2018021709.
Rolfes V, Ribeiro LS, Hawwari I, Böttcher L, Rosero N, Maasewerd S, Santos MLS, Próchnicki T, Silva CMS, Wanderley CWS, Rothe M, Schmidt SV, Stunden HJ, Bertheloot D, Rivas MN, Fontes CJ, Carvalho LH, Cunha FQ, Latz E, Arditi M, Franklin BS. Platelets fuel the inflammasome activation of innate immune cells. Cell Rep. 2020;31(6): 107615. https://doi.org/10.1016/j.celrep.2020.107615.
Gupta N, Sahu A, Prabhakar A, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci USA. 2017;114(18):4763–8. https://doi.org/10.1073/pnas.1620458114.
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li PF, Yu T, Chu XM. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11(9):776. https://doi.org/10.1038/s41419-020-02985-x.
Singh A, Uzun G, Bakchoul T. Primary immune thrombocytopenia: novel insights into pathophysiology and disease management. J Clin Med. 2021;10(4):789. https://doi.org/10.3390/jcm10040789.
Wang S, Liu Y, Li G, Feng Q, Hou M, Peng J. Reduced intracellular antioxidant capacity in platelets contributes to primary immune thrombocytopenia via ROS-NLRP3-caspase-1 pathway. Thromb Res. 2021;199:1–9. https://doi.org/10.1016/j.thromres.2020.12.008.
Lv Y, Ruan G, Liu Y, Cui D, Zhao Y, Yan C, Lv M, Xu D, Mao Y, Cao J, Jin J, Xie J. Aberrant expression of NLRP3, NLRC4 and NLRP6 inflammasomes in patients with primary immune thrombocytopenia. Thromb Res. 2019;176:101–3. https://doi.org/10.1016/j.thromres.2019.02.020.
Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21(6):1918. https://doi.org/10.3390/ijms21061918.
Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130(16):1795–9. https://doi.org/10.1182/blood-2017-03-745349.
Campos J, Ponomaryov T, De Prendergast A, et al. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021;5(9):2319–24. https://doi.org/10.1182/bloodadvances.2020003377.
Zhang Y, Cui J, Zhang G, et al. Inflammasome activation promotes venous thrombosis through pyroptosis. Blood Adv. 2021;5(12):2619–23. https://doi.org/10.1182/bloodadvances.2020003041.
Gomes T, Várady CBS, Lourenço AL, Mizurini DM, Rondon AMR, Leal AC, Gonçalves BS, Bou-Habib DC, Medei E, Monteiro RQ. IL-1β blockade attenuates thrombosis in a neutrophil extracellular trap-dependent breast cancer model. Front Immunol. 2019;4(10):2088. https://doi.org/10.3389/fimmu.2019.02088.
Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45. https://doi.org/10.1038/nri3345.
Nader E, Romana M, Connes P. The red blood cell-inflammation vicious circle in sickle cell disease. Front Immunol. 2020;13(11):454. https://doi.org/10.3389/fimmu.2020.00454.
de Freitas DV, Leal VNC, Fernandes FP, Souza CRL, Figueiredo MS, Pontillo A. Genetic contribution and functional impairment of inflammasome in sickle cell disease. Cytokine. 2022;149: 155717. https://doi.org/10.1016/j.cyto.2021.155717.
Vats R, Brzoska T, Bennewitz MF, Jimenez MA, Pradhan-Sundd T, Tutuncuoglu E, Jonassaint J, Gutierrez E, Watkins SC, Shiva S, Scott MJ, Morelli AE, Neal MD, Kato GJ, Gladwin MT, Sundd P. Platelet extracellular vesicles drive inflammasome-IL-1β-dependent lung injury in sickle cell disease. Am J Respir Crit Care Med. 2020;201(1):33–46. https://doi.org/10.1164/rccm.201807-1370OC.
Mendonça R, Silveira AA, Conran N. Red cell DAMPs and inflammation. Inflamm Res. 2016;65(9):665–78. https://doi.org/10.1007/s00011-016-0955-9.
Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19(4):197–214. https://doi.org/10.1038/s41568-019-0123-y.
Ratajczak MZ, Bujko K, Cymer M, Thapa A, Adamiak M, Ratajczak J, Abdel-Latif AK, Kucia M. The Nlrp3 inflammasome as a “rising star” in studies of normal and malignant hematopoiesis. Leukemia. 2020;34(6):1512–23. https://doi.org/10.1038/s41375-020-0827-8.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
Shroff GS, Truong MT, Carter BW, Benveniste MF, Kanagal-Shamanna R, Rauch G, Viswanathan C, Boddu PC, Daver N, Wu CC. Leukemic involvement in the thorax. Radiographics. 2019;39(1):44–61. https://doi.org/10.1148/rg.2019180069.
Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–87. https://doi.org/10.1182/blood-2008-07-172007.
McReynolds LJ, Savage SA. Pediatric leukemia susceptibility disorders: manifestations and management. Hematol Am Soc Hematol Educ Program. 2017;2017(1):242–50. https://doi.org/10.1182/asheducation-2017.1.242.
Fadeel B, Garwicz D, Carlsson G, Sandstedt B, Nordenskjöld M. Kostmann disease and other forms of severe congenital neutropenia. Acta Paediatr. 2021;110(11):2912–20. https://doi.org/10.1111/apa.16005.
Gluzman DF, Sklyarenko LM, Zavelevich MP, Koval SV, Ivanivska TS, Rodionova NK. Overview on association of different types of leukemias with radiation exposure. Exp Oncol. 2015;37(2):89–93.
Poynter JN, Richardson M, Blair CK, Roesler MA, Hirsch BA, Nguyen P, Cioc A, Warlick E, Cerhan JR, Ross JA. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134–40. https://doi.org/10.1016/j.canep.2015.12.005.
Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, Silver RT, Weiss RB, Moore JO, Schiffer CA, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. J Natl Cancer Inst. 1993;85(24):1994–2003. https://doi.org/10.1093/jnci/85.24.1994.
Wei J, Wang H, Wang H, Wang B, Meng L, Xin Y, Jiang X. The role of NLRP3 inflammasome activation in radiation damage. Biomed Pharmacother. 2019;118: 109217. https://doi.org/10.1016/j.biopha.2019.109217.
Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. https://doi.org/10.1038/nm.2279.
Buscetta M, Di Vincenzo S, Miele M, Badami E, Pace E, Cipollina C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages. FASEB J. 2020;34(1):1819–32. https://doi.org/10.1096/fj.201901239R.
Zhong C, Wang R, Hua M, Zhang C, Han F, Xu M, Yang X, Li G, Hu X, Sun T, Ji C, Ma D. NLRP3 inflammasome promotes the progression of acute myeloid leukemia Via il-1β pathway. front immunol. 2021;15(12): 661939. https://doi.org/10.3389/fimmu.2021.661939.
Hamarsheh S, Osswald L, Saller BS, Unger S, De Feo D, Vinnakota JM, Konantz M, Uhl FM, Becker H, Lübbert M, Shoumariyeh K, Schürch C, Andrieux G, Venhoff N, Schmitt-Graeff A, Duquesne S, Pfeifer D, Cooper MA, Lengerke C, Boerries M, Duyster J, Niemeyer CM, Erlacher M, Blazar BR, Becher B, Groß O, Brummer T, Zeiser R. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Nat Commun. 2020;11(1):1659. https://doi.org/10.1038/s41467-020-15497-1.
Liu Q, Hua M, Zhang C, Wang R, Liu J, Yang X, Han F, Hou M, Ma D. NLRP3-activated bone marrow dendritic cells play antileukemic roles via IL-1β/Th1/IFN-γ in acute myeloid leukemia. Cancer Lett. 2021;520:109–20. https://doi.org/10.1016/j.canlet.2021.06.014.
Wang H, Hua M, Wang S, Yu J, Chen C, Zhao X, Zhang C, Zhong C, Wang R, He N, Hou M, Ma D. Genetic polymorphisms of IL-18 rs1946518 and IL-1β rs16944 are associated with prognosis and survival of acute myeloid leukemia. Inflamm Res. 2017;66(3):249–58. https://doi.org/10.1007/s00011-016-1012-4.
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6): e577. https://doi.org/10.1038/bcj.2017.53.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. https://doi.org/10.1182/blood-2016-01-643569.
Uzan B, Poglio S, Gerby B, et al. Interleukin-18 produced by bone marrow-derived stromal cells supports T-cell acute leukaemia progression. EMBO Mol Med. 2014;6(6):821–34. https://doi.org/10.1002/emmm.201303286.
Zhang C, Han F, Yu J, Hu X, Hua M, Zhong C, Wang R, Zhao X, Shi Y, Ji C, Ma D. Investigation of NF-κB-94ins/del ATTG and CARD8 (rs2043211) Gene Polymorphism in Acute Lymphoblastic Leukemia. Front Endocrinol (Lausanne). 2019;2(10):501. https://doi.org/10.3389/fendo.2019.00501.
Minciacchi VR, Kumar R, Krause DS. Chronic myeloid leukemia: a model disease of the past, present and future. Cells. 2021;10(1):117. https://doi.org/10.3390/cells10010117.
Zhang B, Chu S, Agarwal P, Campbell VL, Hopcroft L, Jørgensen HG, Lin A, Gaal K, Holyoake TL, Bhatia R. Inhibition of interleukin-1 signaling enhances elimination of tyrosine kinase inhibitor–treated CML stem cells. Blood. 2016;128:2671–82. https://doi.org/10.1182/blood-2015-11-679928.
Xu Z, Wang H, Wei S, Wang Z, Ji G. Inhibition of ER stress-related IRE1α/CREB/NLRP1 pathway promotes the apoptosis of human chronic myelogenous leukemia cell. Mol Immunol. 2018;101:377–85. https://doi.org/10.1016/j.molimm.2018.07.002.
Zhang A, Yu J, Yan S, Zhao X, Chen C, Zhou Y, Zhao X, Hua M, Wang R, Zhang C, Zhong C, He N, Ji C, Ma D. The genetic polymorphism and expression profiles of NLRP3 inflammasome in patients with chronic myeloid leukemia. Hum Immunol. 2018;79(1):57–62. https://doi.org/10.1016/j.humimm.2017.10.013.
Haseeb M, Anwar MA, Choi S. Molecular Interactions between innate and adaptive immune cells in chronic lymphocytic leukemia and their therapeutic implications. Front Immunol. 2018;26(9):2720. https://doi.org/10.3389/fimmu.2018.02720.
Salaro E, Rambaldi A, Falzoni S, Amoroso FS, Franceschini A, Sarti AC, Bonora M, Cavazzini F, Rigolin GM, Ciccone M, Audrito V, Deaglio S, Pelegrin P, Pinton P, Cuneo A, Di Virgilio F. Involvement of the P2X7-NLRP3 axis in leukemic cell proliferation and death. Sci Rep. 2016;25(6):26280. https://doi.org/10.1038/srep26280.
de Leval L, Jaffe ES. Lymphoma classification. Cancer J. 2020;26(3):176–85. https://doi.org/10.1097/PPO.0000000000000451.
Zhao X, Zhang C, Hua M, Wang R, Zhong C, Yu J, Han F, He N, Zhao Y, Liu G, Zheng N, Ji C, Ma D. NLRP3 inflammasome activation plays a carcinogenic role through effector cytokine IL-18 in lymphoma. Oncotarget. 2017;8(65):108571–83. https://doi.org/10.18632/oncotarget.21010.
Huanosta-Murillo E, Alcántara-Hernández M, Hernández-Rico B, et al. NLRP3 regulates IL-4 expression in TOX+ CD4+ T cells of cutaneous T cell lymphoma to potentially promote disease progression. Front Immunol. 2021;12:668369. https://doi.org/10.3389/fimmu.2021.668369.
Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Végran F, Ghiringhelli F. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16(8):859–70. https://doi.org/10.1038/ni.3202.
Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H, Zou J, Wang Y, Li G, Sun T, Li J, Ma D, Ye J, Ji C. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2021;28(497):178–89. https://doi.org/10.1016/j.canlet.2020.10.024.
Baldini C, Santini E, Rossi C, Donati V, Solini A. The P2X7 receptor-NLRP3 inflammasome complex predicts the development of non-Hodgkin’s lymphoma in Sjogren’s syndrome: a prospective, observational, single-centre study. J Intern Med. 2017;282(2):175–86. https://doi.org/10.1111/joim.12631.
Costes V, Portier M, Lu ZY, Rossi JF, Bataille R, Klein B. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production. Br J Haematol. 1998;103(4):1152–60. https://doi.org/10.1046/j.1365-2141.1998.01101.x.
Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, Donovan KA. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91(6):571–4. https://doi.org/10.1002/ajh.24352.
Zhao X, Hua M, Yan S, Yu J, Han F, Zhong C, Wang R, Zhang C, Hou M, Ma D. The genetic polymorphisms of NLRP3 Inflammasome associated with T helper cells in patients with multiple myeloma. J Immunol Res. 2018;23(2018):7569809. https://doi.org/10.1155/2018/7569809.
Li Y, Li N, Yan Z, Li H, Chen L, Zhang Z, Fan G, Xu K, Li Z. Dysregulation of the NLRP3 inflammasome complex and related cytokines in patients with multiple myeloma. Hematology. 2016;21(3):144–51. https://doi.org/10.1179/1607845415Y.0000000029.
Cazzola M. Myelodysplastic syndromes. N Engl J Med. 2020;383(14):1358–74. https://doi.org/10.1056/NEJMra1904794.
Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, Zhang Q, Irvine BA, Cluzeau T, Sallman DA, Padron E, Komrokji R, Sokol L, Coll RC, Robertson AA, Cooper MA, Cleveland JL, O’Neill LA, Wei S, List AF. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960–75. https://doi.org/10.1182/blood-2016-07-730556.
Sallman DA, Cluzeau T, Basiorka AA, List A. Unraveling the pathogenesis of MDS: the NLRP3 inflammasome and pyroptosis drive the MDS phenotype. Front Oncol. 2016;6:151. https://doi.org/10.3389/fonc.2016.00151.
Yin C, He N, Li P, Zhang C, Yu J, Hua M, Ji C, Ma D. Polymorphisms of Interlukin-1β rs16944 confer susceptibility to myelodysplastic syndromes. Life Sci. 2016;15(165):109–12. https://doi.org/10.1016/j.lfs.2016.09.019.
Braun LM, Zeiser R. Immunotherapy in myeloproliferative diseases. Cells. 2020;9(6):1559. https://doi.org/10.3390/cells9061559.
Battista Di, Valeria, et al. Genetics and pathogenetic role of inflammasomes in Philadelphia negative chronic myeloproliferative neoplasms: a narrative review. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22020561.
Liew EL, Araki M, Hironaka Y, et al. Identification of AIM2 as a downstream target of JAK2V617F. Exp Hematol Oncol. 2016;5:2. https://doi.org/10.1186/s40164-016-0032-7.
Höchsmann B, Murakami Y, Osato M, et al. Complement and inflammasome overactivation mediates paroxysmal nocturnal hemoglobinuria with autoinflammation. J Clin Invest. 2019;129(12):5123–36. https://doi.org/10.1172/JCI123501.
Brodsky RA. Paroxysmal nocturnal hemoglobinuria without GPI-anchor deficiency. J Clin Invest. 2019;129(12):5074–6. https://doi.org/10.1172/JCI131647.
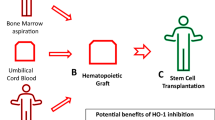
Passweg JR, Baldomero H, Basak GW, Chabannon C, Corbacioglu S, Duarte R, Kuball J, Lankester A, Montoto S, de Latour RP, Snowden JA, Styczynski J, Yakoub-Agha I, Arat M, Mohty M, Kröger N. European Society for Blood and Marrow Transplantation (EBMT) The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transpl. 2019;54(10):1575–85. https://doi.org/10.1038/s41409-019-0465-9.
Xiao J, Wang C, Yao JC, Alippe Y, Yang T, Kress D, Sun K, Kostecki KL, Monahan JB, Veis DJ, Abu-Amer Y, Link DC, Mbalaviele G. Radiation causes tissue damage by dysregulating inflammasome-gasdermin D signaling in both host and transplanted cells. PLoS Biol. 2020;18(8): e3000807. https://doi.org/10.1371/journal.pbio.3000807.
Adamiak M, Bujko K, Cymer M, et al. Novel evidence that extracellular nucleotides and purinergic signaling induce innate immunity-mediated mobilization of hematopoietic stem/progenitor cells [published correction appears in Leukemia]. Leukemia. 2018;32(9):1920–31. https://doi.org/10.1038/s41375-018-0122-0.
Ringden O, Hassan Z, Karlsson H, Olsson R, Omazic B, Mattsson J, Remberger M. Granulocyte colony-stimulating factor induced acute and chronic graft-versus-host disease. Transplantation. 2010;90(9):1022–9. https://doi.org/10.1097/TP.0b013e3181f585c7.
Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, Rossignol DP, Ferrara JL. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107(12):1581–9. https://doi.org/10.1172/JCI12156.
Jankovic D, Ganesan J, Bscheider M, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210(10):1899–910. https://doi.org/10.1084/jem.20130084.
Vanhaver C, van der Bruggen P, Bruger AM. MDSC in mice and men: mechanisms of immunosuppression in cancer. J Clin Med. 2021;10(13):2872. https://doi.org/10.3390/jcm10132872.
Koehn BH, Saha A, McDonald-Hyman C, Loschi M, Thangavelu G, Ma L, Zaiken M, Dysthe J, Krepps W, Panthera J, Hippen K, Jameson SC, Miller JS, Cooper MA, Farady CJ, Iwawaki T, Ting JP, Serody JS, Murphy WJ, Hill GR, Murray PJ, Bronte V, Munn DH, Zeiser R, Blazar BR. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood. 2019;134(19):1670–82. https://doi.org/10.1182/blood.2019001950.
Takahashi H, Okayama N, Yamaguchi N, et al. Associations of interactions between NLRP3 SNPs and HLA mismatch with acute and extensive chronic graft-versus-host diseases. Sci Rep. 2017;7(1):13097. https://doi.org/10.1038/s41598-017-13506-w.
Granell M, Urbano-Ispizua A, Pons A, Aróstegui JI, Gel B, Navarro A, Jansa S, Artells R, Gaya A, Talarn C, Fernández-Avilés F, Martínez C, Rovira M, Carreras E, Rozman C, Juan M, Yagüe J, Montserrat E, Monzó M. Common variants in NLRP2 and NLRP3 genes are strong prognostic factors for the outcome of HLA-identical sibling allogeneic stem cell transplantation. Blood. 2008;112(10):4337–42. https://doi.org/10.1182/blood-2007-12-129247.
Land WG. Transfusion-related acute lung injury: the work of DAMPs. Transfus Med Hemother. 2013;40(1):3–13. https://doi.org/10.1159/000345688.
Gibb DR, Calabro S, Liu D, Tormey CA, Spitalnik SL, Zimring JC, Hendrickson JE, Hod EA, Eisenbarth SC. The Nlrp3 inflammasome does not regulate alloimmunization to transfused red blood cells in mice. EBioMedicine. 2016;9:77–86. https://doi.org/10.1016/j.ebiom.2016.06.008.
Chong F, Rooks KM, Flower RL, Dean MM. Soluble mediators in packed red blood cells augment lipopolysaccharide-induced monocyte interleukin-1β production. Vox Sang. 2020;115(7):562–9. https://doi.org/10.1111/vox.12915.
Sippert EÂ, Visentainer JE, Alves HV, Rodrigues C, Gilli SC, Addas-Carvalho M, Saad ST, Costa FF, Castilho L. Red blood cell alloimmunization in patients with sickle cell disease: correlation with HLA and cytokine gene polymorphisms. Transfusion. 2017;57(2):379–89. https://doi.org/10.1111/trf.13920.
Liu D, Xu X, Dai Y, et al. Blockade of AIM2 inflammasome or α1-AR ameliorates IL-1β release and macrophagemediated immunosuppression induced by CAR-T treatment. J Immunother Cancer. 2021;9: e001466. https://doi.org/10.1136/jitc-2020-001466.
Weber ANR. Targeting the NLRP3 Inflammasome via BTK. Front Cell Dev Biol. 2021;25(9): 630479. https://doi.org/10.3389/fcell.2021.630479.
Funding
VFD-Conselho Nacional de desenvolvimento científico e tecnológico (CNPq)- Fellowship (162577/2015–0). VNCL-Fundação de Amparo à pesquisa do estado de São Paulo (FAPESP)- Postdoctoral Fellowship (2020/15323–3). AP-Conselho Nacional de desenvolvimento científico e tecnológico (CNPq)-Excellence program (302206/2019–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Freitas Dutra, V., Leal, V.N.C. & Pontillo, A. The inflammasomes: crosstalk between innate immunity and hematology. Inflamm. Res. 71, 1403–1416 (2022). https://doi.org/10.1007/s00011-022-01646-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01646-3