Abstract
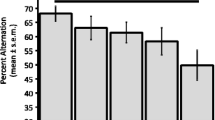
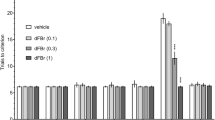
To study the effects of repeated ketamine administration on central muscarinic acetylcholine receptors (mAchRs), ddY male mice were administered subcutaneous doses of 25 mg/kg ketamine every 3 days for a total of five times. Receptor binding assays of mAchR were carried out in the forebrain (FB), cerebellum (CB) and brainstem (BS), using [3H]quinuclidinyl benzilate ([3H]QNB) as a ligand. In addition, we examined whether repeated ketamine (12.5, 25 and 50 mg/kg) or saline (five times) could modify the hyperlocomotion induced by scopolamine (0.5 mg/kg, SC) (a muscarinic antagonist), using a behavior-pharmacological technique. Repeating the ketamine administration resulted in a significant increase in the receptor density value (Bmax) for [3H]QNB only in FB, dependent on the numbers of administrations (1270 ± 33 fmol/mg protein for a single dose, 1620 ± 59 for four treatments, 1738 ± 70 for five treatments without any change in apparent affinity (defined as the reciprocal of the dissociation constant) (Kd). A competitive inhibition study of repeated (5 times) administration of ketamine failed to detect any subtype-specific changes in mAchRs. Repeated ketamine administration reduced the scopolamine-induced hyperlocomotion in a doserelated way, and the changes were significant at 50 mg/kg. Our results suggest that repeated ketamine administration produces an up-regulation of mAchRs, and this change may be associated with altered Ach transmission in the central nervous system.
Similar content being viewed by others
References
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectivity reduce excitation of central mammalian neurons byN-methyl-aspartate. Br J Pharmacol 79:565–575
Aronstan RS, Narayanan L, Wanga DA (1983) Ketamine inhibition of ligand binding to cholinergic receptors and ion channels. Eur J Pharmacol 78:367–370
Ben-Barak J, Dudai Y (1980) Scopolamine induces an increase in muscarinic receptor level in rat hippocampus. Brain Res 193:309–313
Bennet JA, Bullimore JA (1973) The use of ketamine hydrochloride anesthesia for radiotherapy in young children. Br J Anaesth 45:197–201
Berstein G, Haga T (1990) Molecular aspects of muscarinic receptors. In: Osborne NN (ed) Current aspects of the neurosciences 1. Macmillan Press, New York, pp 245–284
Berstein G, Haga T, Ichiyama A (1989) Effect of the lipid on the differential affinity of purified cerebral and atrial environment muscarinic acetylcholine receptors for pirenzepine. Mol Pharmacol 36:601–607
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein dye binding. Anal Biochem 72:248–254
Cheng YC, Prusoff WH (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which cause 50 per cent inhibition (IC50) of an enzymatic reaction. Biochemical Pharmacol 22:3099–3108
Church J, Zeman S, Lodge D (1988) The neuroprotective action of ketamine and MK-801 after transient cerebral ischemia in rats. Anesthesiology 69:702–709
Cochen ML, Chan SL, Way WL, Trevor AJ (1973) Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology 39:370–376
Durkin TP, Hashem-Zaden H, Villareal JE (1983) Genotype variation in the dopaminergic inhibitory control of striatal and hippocampal cholinergic activity in mice. Pharmacol Biochem Behav 19:63–70
El-Fakahany EE, Cioffi CL, Abdellatif MM, Miller MM (1986) Competitive interaction of pirenzepine with rat brain muscarinic acetylcholine receptors. Eur J Pharmacol. 131:237–247
Fleming N, Mellow L, Bhullar D (1992) Regulation of the cAMP signal transduction pathway by protein kinase C in rat submandibular cells. Eur J Physiol 421:82–89
Gandolfi O, Dall'Olio R, Roncada P, Montanaro N (1990) NMDA antagonists interact with 5-HT-stimulated phosphatidylinositol metabolism and impair passive avoidance retention in the rat. Neurosci Lett 113:304–308
Gjessing J (1968) Ketamine (CI 581) in clinical anesthesia. Acta Anaesthesiol Scand 12:15–21
Hadcock JR, Malbon CC (1991) Regulation of receptor expression by agonists: transcriptional and post-transcriptional controls. Trends Neurol Sci 14:242–247
Hata F, Takeyasu K, Morikawa Y, Lai RT, Ishida H, Yoshida H (1980) Specific changes in the cholinergic system in guinea-pig vas deferens after denervation. J Pharmacol Exp Ther 215:716–722
Hille G (1992) G protein-coupled mechanisms and nervous signaling. Neuron 9:187–195
Houslay M (1991) Crosstalk: a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem 195:9–27
Huganir RL, Greengard P (1990) Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron 5:555–567
Hulme RI, Birdsall NJM, Buckley NJ (1990) Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol 30:633–673
Irifune M, Shimizu T, Nomoto M (1991) Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 40:399–407
Majocha R, Baldessarini RJ (1984) Tolerance to an anticholinergic agent is paralleled by increased binding to muscarinic receptors in rat brain and increased behavioral response to a centrally active cholinomimetic. Life Sci 35:2247–2255
Mei L, Roeske WR, Yamamura HI (1989) Molecular pharmacology of muscarinic receptor heterogeneity. Life Sci 45:1831–1851
Oungazaal A, Nieoullon A, Amalric M (1993) Effects of dopamine D1 and D2 receptor blockade on MK-801-induced hyperlocomotion in rats. Psychopharmacology 111:427–434
Raiteri M, Marachi M, Paudice P (1990) Presynaptic muscarinic receptors in the central nervous system. Ann NY Acad Sci 604:113–129
Roth RH, Wolf ME, Deutch AY (1987) Neurochemistry of midbrain dopamine systems. In: Meltzer HY (ed) Psychopharmacology. Raven Press, New York, pp 81–94
Ryder S, Way WL, Trevor AJ (1978) Comparative pharmacology of the optical isomers of ketamine in mice. Eur J Pharmacol 49:15–23
Saito S, Komiya Y, Igarashi M (1991) Muscarinic acetylcholine receptors are expressed and enriched in growth cone membranes isolated from fetal and neonatal rat forebrain: pharmacological demonstration and characterization. Neuroscience 45:735–745
Scatchard G (1949) The attraction of protein for small moelcules and ions. Ann NY Acad Sci 51:660–672
Shimerlik MI (1989) Structure and regulation of muscarinic receptors. Annu Rev Physiol 51:217–227
Shukla VK, Turndorf H, Puig MM, Bansinath M (1991) Ketamine-induced inhibition of cyclic AMP in mice brain: role of pertussis toxin-sensitive G proteins. Ann NY Acad Sci 625:44–447
Smith DJ, Pekae GM, Martin LL (1980) The interaction of ketamine with the opiate receptor. Life Sci 26:789–795
Snell LD, Mueller ZL, Gannon RL, Silverman PB, Johnson KM (1984) A comparison between classes of drugs having phencyclidine-like behavioral properties on dopamine efflux in vitro and dopamine metabolism in vivo. J Pharmacol Exp Ther 231:261–269
Thomson AM, Wes DC, Lodge D (1985) AnN-metylasparate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature 313:479–481
Uchihashi Y, Kuribara H, Morita T, Fujita T (1993) The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Jpn J Pharmacol 61:149–151
Uchihashi Y, Kuribara H, Isa Y, Morita T, Sato T (1994) The disruptive effects of ketamine on passive avoidance learning in mice: involvement of dopaminergic mechanism. Psychopharmacology 116:40–44
Verma A, Kulkarni SK (1991) Modulation of MK-801 response by dopaminergic agents in mice. Psychopharmacology 107:432–436
Wood PL, Steel DS, McPherson SE, Cheney DL, Lehmann J (1987) Antagonism ofN-methyl-d-asparate (NMDA) evoked increases in cerebellar cGMP and striatal Ach release by phencyclidine (PCP) receptor agonists: evidence for possible allosteric coupling of NMDA and PCP receptors. Can J Physiol Pharmacol 65:1923–1927
Yamamura T, Harada K, Okamura A, Kenmotsu O (1990) Is the site of ketamine anesthesiaN-methyl-d-asparate receptor? Anesthesiology 72:704–710
Ylitalo P, Saarnivaar L, Ahtee L (1976) Effects of ketamine anesthesia on the content of monoamines and their metabolites in the rat brain. Acta Anaesth Scand 20:216–220
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morita, T., Hitomi, S., Saito, S. et al. Repeated ketamine administration produces up-regulation of muscarinic acetylcholine receptors in the forebrain, and reduces behavioral sensitivity to scopolamine in mice. Psychopharmacology 117, 396–402 (1995). https://doi.org/10.1007/BF02246210
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02246210