Summary
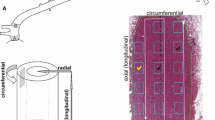
The normal development of elastin fibers in the thoracic aorta was studied in fetal, young, and adult monkeys. Tissue was examined by scanning electron microscopy (SEM) after NaOH treatment and by transmission electron microscopy (TEM). The NaOH treatment of fixed tissues effectively removed collagen fibers and enabled three-dimensional visualization of the elastin fibers. In intact fetal aortae, the internal elastic lamina (IEL) was situated immediately beneath the endothelium. This IEL consisted of superficial, longitudinally arranged bundles of elastin fibrils and an underlying solid sheet containing round fenestrations. In neonates, diffuse intimal thickening was observed. In the young and young-adult monkeys, the aortae exhibited intimal thickening with slender but split IEL. One of the most important findings of this study was that elastin fibers in the intimal thickening, as well as smooth muscle cells, ran in a longitudinal fashion. This was in contrast with the elastic laminae of the media which were mainly oriented circumferentially. Subendothelial elastin fibers in this intimal thickening combined with longitudinally arranged microfibrils which formed close associations with endothelial stress fibers. In some adult monkey aortae with well-developed intimal thickening, a complex meshwork of slender elastin fibers was also found beneath the endothelium. The development of the intimal elastin fibers is discussed in relation to hemodynamic forces.
Similar content being viewed by others
References
Ross R, Bornstein P (1969) The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol 40:366–381
Ross R (1973) The elastic fiber: A review. J Histochem Cytochem 21:199–208
Sandberg LB, Soskel NT, Leslie JG (1981) Elastin structure, biosynthesis, and relation to disease states. New Engl J Med 304:566–579
Rosenbloom J (1987) Elastin: An overview. Method Enzymol 144:172–196
Rojkind M (1973) Molecular structure of the fibrous components of the connective tissue. II. Elastin. In: Pérez-Tamayo R, Rojkind M (eds) Molecular pathology of connective tissue. Dekker, New York, pp 62–76
Fawcett DW (1986) A textbook of histology. Saunders, Philadelphia, pp 367–405
Clark JM, Glagov S (1985) Transmural organization of the arterial media: The lamellar unit revisited. Arteriosclerosis 5:19–34
Movat HZ, More RH, Haust MD (1958) The diffuse intimal thickening of the human aorta with aging. Am J Pathol 34:1023–1031
Smith EB (1974) Acid glycosaminoglycan, collagen and elastin content of normal artery, fatty streaks and plaques. Adv Exp Med Biol 43:125–139
Gansler HN, Weiss H, Wenisch HJC, Noetzel B, Pretzsch U (1979) Intimal thickening of human femoral arteries with special regard to elastin. Atherosclerosis 34:167–191
Geer JC, Haust MD (1972) Smooth muscle cells in atherosclerosis. Monogr Atheroscler 2:1–140
Wasano K, Yamamoto T (1983) Tridimensional architecture of elastic tissue in the rat aorta and femoral artery — A scanning electron microscope study. J Electron Microsc 32:33–44
Ushiki T, Murakumo M (1991) Scanning electron microscopic studies of tissue elastin components exposed by a KOH-collagenase or simple KOH digestion method. Arch Histol Cytol 54:427–436
Song SH, Roach MR (1984) Comparison of fenestrations in internal elastic laminae of canine thoracic and abdominal aortas. Blood Vessels 21:90–97
Dunmore PJ, Song SH, Roach MR (1990) A comparison of the size of fenestrations in the internal elastic lamina of young and old porcine aortas as seen with the scanning electron microscope. Can J Physiol Pharmacol 68:139–143
Ushiki T (1992) Preserving the original architecture of elastin components in the formic acid-digested aorta by an alternative procedure for scanning electron microscopy. J Electron Microsc 41:60–63
Kajikawa K, Yamaguchi T, Katsuda S, Miwa A (1975) An improved electron stain for elastic fibers using tannic acid. J Electron Microsc 24:287–289
Inoue T, Osatake H, Takahashi H (1989) A new freezedrying instrument using t-butyl alcohol for scanning electron microscopy. J Electron Microsc 38:246–249
Roach MR, Song SH (1988) Arterial elastin as seen with scanning electron microscopy: A review. Scanning Microsc 2:993–1004
Miller BG, Woods RI, Bohlen HG, Evan AP (1982) A new morphological procedure for viewing microvessels: A scanning electron microscopic study of the vasculature of small intestine. Anat Rec 203:493–503
Carnes WH, Hart ML, Hodgkin NM (1976) Conformation of aortic elastin revealed by scanning electronmicroscopy of dissected surfaces. In: Sandberg LB, Gray WR, Franzblau C (eds) Elastin and elastic tissue. Plenum, New York, pp 61–70
Campbell GR, Campbell JH (1985) Recent advances in molecular pathology: Smooth muscle cell phenotypic changes in arterial wall homeostasis. Exp Mol Pathol 42:139–162
Ross R (1986) The pathogenesis of atherosclerosis-An update. New Engl J Med 314:488–500
Buck RC (1979) The longitudinal orientation of structures in the subendothelial space of rat aorta. Am J Anat 156:1–14
Wolinsky H, Glagov S (1964) Structural basis for the static mechanical properties of the aortic media. Circ Res 14:400–413
Smith P (1976) A comparison of the orientation of elastin fibers in the elastic laminae of the pulmonary trunk and aorta of rabbits using the scanning electron microscope. Lab Invest 35:525–529
Yohro T, Burnstock G (1973) Filament bundles and contractility of endothelial cells in coronary arteries. Z Zellforsch 138:85–95
Wong AJ, Pollard TD (1983) Actin filament stress fibers in vascular endothelial cells in vivo. Science 219:867–869
White GE, Fujiwara K (1986) Expression and intracellular distribution of stress fibers in aortic endothelium. J Cell Biol 103:63–70
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sato, F., Shimada, T., Kitamura, H. et al. Changes in morphology of elastin fibers during development of the tunica intima of monkey aorta. Heart Vessels 9, 140–147 (1994). https://doi.org/10.1007/BF01745239
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01745239