Abstract
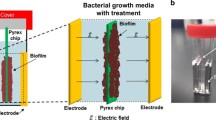
The combined use of antibiotics with low levels of electrical current has been reported to be more effective in controlling biofilms (the bioelectric effect) than antibiotics alone. An electrical colonisation cell was designed to study the effect of antibiotics on biofilms formed on a dialysis membrane away from the electrode surface. To avoid the electrochemical generation of toxic products,Pseudomonas aeruginosa biofilms were formed in minimal salts medium that excluded chloride-containing compounds. Under these conditions, electrical currents of up to 20 mA cm−2 did not prevent biofilm formation or have any detrimental effect on an established biofilm. Tobramycin alone at concentrations of 10 μg ml−1 did not affect the biofilm, but were significantly enhanced by 9 mA cm−2. The effect of tobramycin concentrations of 25 μg ml−1 were enhanced by a 15 mA cm−2 electrical current. In both cases higher levels of electrical current, up to 20 mA cm−2, did not further enhance the effect of the antibiotic. The possible mechanisms of action of the bioelectric effect have been reported to involve electrophoresis, iontophoresis and electroporesis, thus overcoming the biofilm biomass and cell wall barriers. Our results suggest that other factors may also be important, such as the metabolic activity and growth rate of the bacteria. Such factors may be critical in maximising antibiotic efficacy.
Similar content being viewed by others
References
Anwar H and JW Costerton. 1990. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells ofPseudomonas aeruginosa. Antimicrob Agents Chemother 34: 1666–1671.
Baddour LM, LP Barker, GD Christensen, JT Parisi and WA Simpson. 1990. Phenotypic variation ofStaphylococcus epidermidis in infection of transvenous endocardial pacemaker electrodes. J Clin Microbiol 28: 676–679.
Blenkinsopp SA, AE Khoury and JW Costerton. 1992. Electrical enhancement of biocide efficacy againstPseudomonas aeruginosa biofilms. Appl Environ Microbiol 58: 3770–3773.
Brown MRW and P Williams. 1985. Influence of substrated limitation and growth phase on sensitivity to antimicrobial agents. J Antimicrob Chemother 15 (Suppl): 7–14.
Brown ML and JJ Gauthier. 1993. Cell density and growth phase as factors in the resistance of a biofilm ofPseudomonas aeruginosa (ATCC 27853) to iodine. Appl Environ Microbiol 59: 2320–2322.
Castro AJ, GV Barbosa-Canovas and BG Swanson. 1993. Microbial inactivation of foods by pulsed electrical fields. J Food Processing Preservation 17: 47–73.
Costerton JW, B Ellis, K Lam, F Johnson and AE Khoury. 1994. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother 38: 2803–2809.
Davis CP, S Weinberg, MD Anderson, GM Rao and MM Warren. 1989. Effects of microamperage, medium, and bacterial concentration on iontophoretic killing of bacteria in fluid. Antimicrob Agents Chemother 33: 442–447.
Davis CP, N Wagle, MD Anderson and MM Warren. 1991. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob Agents Chemother 35: 2131–2134.
Davis CP, N Wagle, MD Anderson and MM Warren. 1992. Iontophoresis generates an antimicrobial effect that remains after iontophoresis ceases. Antimicrob Agents Chemother 36: 2552–2555.
Davis CP, ME Shirtliff, NM Trieff, SL Hoskins and MM Warren. 1994. Quantification, qualification, and microbial killing efficiencies of antimicrobial chlorine-based substances produced by iontophoresis. Antimicrob Agents Chemother 38: 2768–2774.
Eng RHK, FT Padberg, SM Smith, EN Tan and CE Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35: 1824–1828.
Evans DJ, MRW Brown, DG Allison and P Gilbert. 1990. Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicrob Chemother 25: 585–591.
Farber BF, MH Kaplan and AG Clogston. 1990.Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis 161: 37–40.
Franklin TJ and GA Snow. 1989. Biochemistry of Antimicrobial Action. pp 55–72, Chapman and Hall, New York.
Gilbert P and MRW Brown. 1978. Influence of growth rate and nutrient limitation on the gross cellular composition ofPseudomonas aeruginosa and its resistance to 3- and 4-chlorophenol. J Bacteriol 133: 1066–1072.
Gilbert P, MRW Brown and JW Costerton. 1987. Inocula for antimicrobial sensitivity testing: a critical review. J Antimicrob Chemother 20: 147–154.
Gilbert P, DJ Evans, E Evans, IG Duguid and MRW Brown. 1991. Surface characteristics and adhesion ofEscherichia coli andStaphylococcus epidermidis. J Appl Bacteriol 71: 72–77.
Goldmann DA and GB Pier. 1993. Pathogenesis of infections related to intravascular catheterization. Clin Microbiol Rev 6: 176–192.
Gristina AG, JW Costerton and PLJ McGanity. 1984. Bacterial-laden biofilms: a hazard to orthopaedic prostheses. Infect in Surgery 3: 655–662.
Haslett TM, HD Isenberg, E Hilton, V Tucci, BG Kay and EM Vellozzi. 1988. Microbiology of indwelling central intravascular catheters. J Clin Microbiol 26: 696–701.
Hoyle BD, J Jass and JW Costerton. 1990. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother 26: 1–6.
Hoyle BD, J Alcantara and JW Costerton. 1992.Pseudomonas aeruginosa biofilms as a diffusion barrier to piperacillin. Antimicrob Agents Chemother 36: 2054–2056.
Jones RN, AL Gavan and JA Washinton II. 1985. Susceptibility tests: microdilution and macrodilution broth procedures. In: Manual of Clinical Microbiology. 4th edn (Lennette EH, A Balows, WJ Hausler Jr and HS Shadomy, eds), pp 972–977. ASM Press, Washington.
Karchmer AW and GW Gibbons. 1994. Infections of prosthetic heart valves and vascular grafts. In: Infections Associated with Indwelling Medical Devices (Bisno AL and FA Waldvogel, eds), pp 213–250. ASM Press, Washington.
Lappin-Scott HM, JW Costerton and TJ Marrie. 1992. Biofilms and biofouling. In: Encyclopaedia of Microbiology, Vol 1 (Lederberg J, ed), pp 277–284, Academic Press, San Diego.
Lopez-Lopez G, A Pascual and J Perea. 1991. Effect of plastic catheter material on bacterial adherence and viability. J Med Microbiol 34: 349–353.
Marrie TJ and JW Costerton. 1984. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol 19: 687–693.
Matsunaga T, S Nakasono, T Takamuka, JG Burgess, N Nakamura and K Sode. 1992. Disinfection of drinking water by using a novel electrochemical reactor employing carbon-cloth eletrodes. Appl Environ Microbiol 58: 686–689.
Miller MJ and DG Ahearn. 1987. Adherence ofPseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J Clin Microbiol 25: 1392–1397.
Nickel JC, AG Gristina and JW Costerton. 1985. Electron microscopic study of an infected Foley catheter. Can J Surg 28: 50–52.
Nickel JC, JB Wright, I Ruseska, TJ Marrie, C Whitfield, JW Costerton. 1985. Antibiotic resistance ofPseudomonas aeruginosa colonizing a urinary catheter. Eur J Clin Microbiol 4: 213–218.
Okuno K, K Tuchiya, T Ano and M Shoda. 1993. Effect of super high magnetic field on the growth ofEscherichia coli under various medium compositions and temperatures. J Ferment Bioeng 75: 103–106.
Pitt WG, MO McBride, JK Lunceford, RJ Roper and RD Sagers. 1994. Ultrasonic enhancement of antibiotic action on Gram-negative bacteria. Antimicrob Agents Chemother 38: 2577–2582.
Pothakamury UR, GV Barbosa-Canovas and BG Swanson. 1993. Magnetic-field inactivation of microorganisms and generation of biological changes. Food Technol 47: 85–93.
Rajnicek AM, CD McCaig and NAR Gow. 1994. Electric field induced growth ofEnterobacter cloacae, Escherichia coli, andBacillus subtilis cells: implications for mechanisms of galvanotropism and bacterial growth. J Bacteriol 176: 702–713.
Read RR, P Eberwein, MK Dasgupta, SK Grant, K Lam, JC Nickel and JW Costerton. 1989. Peritonitis in peritoneal dialysis: bacterial colonization by biofilm spread along the catheter surface. Kidney Int 35: 614–621.
Sale AJH and WA Hamilton. 1967. Effects of high electrical fields on microorganisms. I. Killing of bacteria and yeasts. Biochim Biophys Acta 148: 781–788.
Sale AJH and WA Hamilton. 1967. Effects of high electrical fields on microorganisms. II. Mechanism of action of the lethal effect. Biochim Biophys Acta 148: 781–788.
Schoenknecht FD, LD Sabath and C Thornsberry. 1985. Susceptibility tests: special tests. In: Manual of Clinical Microbiology. 4th edn (Lennette EH, A Balows, WJ Hausler Jr and HS Shadomy, eds), pp 1000–1008, ASM Press, Washington.
Taber HW, JP Mueller, PF Miller and AS Arrow. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51: 439–457.
Tiruviluamala P and WG Johanson Jr. 1994. Infections Associated with endotracheal intubation and tracheostomy. In: Infections Associated with Indwelling Medical Devices (Bisno AL and FA Waldvogel, eds), pp 135–154, ASM Press, Washington.
Walsh S and HM Lappin-Scott. 1994. Starvation of thermophilic sulphate-reducing bacteria isolated from cold marine environments. In: 94th ASM General Meeting Abstract, p 340, Abstracts N139, May 1994, American Society for Microbiology, Washington.
Widmer AF, R Frei, Z Rajacic and W Zimmerli. 1990. Correlation betweenin vivo andin vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis 162: 96–102.
Widmer AF, A Weistner, R Frei and W Zimmerli. 1991. Killing of nongrowing and adherentEscherichia coli determines drug efficacy in device-related infections. Antimicrob Agents Chemother 35: 741–746.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jass, J., Costerton, J.W. & Lappin-Scott, H.M. The effect of electrical currents and tobramycin onPseudomonas aeruginosa biofilms. Journal of Industrial Microbiology 15, 234–242 (1995). https://doi.org/10.1007/BF01569830
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569830