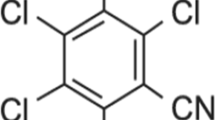
Abstract
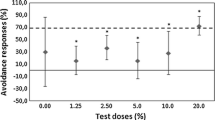
The effects of 24 to 72-hr exposure to fenthion (101–103 ppb) were determined for a fungal community, nitrogen-fixing microbes, and representative meiofaunal and zooplankton invertebrates of a mangrove ecosystem. Also tested were the abilities of a benthic diatom and of fungi to grow in the presence of fenthion. Acute lethal, growth-inhibiting, or processdisrupting effects were not detected for exposures to less than 500 ppb fenthion. Results are compared with the findings of several other investigations of the impact of fenthion and other organophosphorus insecticides on non-target organisms.
Similar content being viewed by others
References
Baarschers, W. H., A. I. Bharath, M. Hazenberg, and J. E. Todd: Fungitoxicity of methoxychlor and fenitrothion and the environmental impact of their metabolites. Can. J. Bot.58, 426 (1980).
Boike, A. H., C. B. Rathburn, C. F. Hallmon, and S. G. Cotterman: Insecticide susceptibility tests ofAedes taeniorhynchus andCulex nigripalpus in Florida, 1974–1976. Mosq. News38, 210 (1978).
Bookhout, C. G., and R. J. Monroe: Effects of malathion on the development of crabs. In F. J. Vernberg, A. Calabrese, F. P. Thurberg, and W. B. Vernberg (eds.): Physiological responses of marine biota to pollutants, p. 3. New York: Academic Press (1977).
Cole, M. A., and A. J. Turgeon: Microbial activity in soil and litter underlying bandane- and calcium arsenate-treated turfgrass. Soil Biol. Biochem.10, 181 (1978).
Congregado, F., D. Simon-Pujol, and A. Juarez: Effect of two organophosphorus insecticides on the phosphate-dissolving soil bacteria. Appl. Environ. Microbiol.37, 169 (1979).
Cooksey, K. E., and B. Cooksey: Growth-influencing substances in sediment extracts from a subtropical wetland: Investigation using a diatom bioassay. J. Phycol.14, 347 (1978).
Cooksey, K. E., B. Cooksey, P. M. Evans, and E. L. Hildebrand: Benthic diatoms as contributors to the carbon cycle in a mangrove community. Tenth European Symposium on Marine Biology (Ostend, Belgium)2, 165 (1975).
Coull, B. C.: Aspects of benthic-pelagic coupling in estuarine systems. In A. Q. White (ed.): Estuarine perspectives, in press. New York: Academic Press (1980).
Derby, S. B., and E. Ruber: Primary production: Depression of oxygen evolution in algal cultures by organophosphorus insecticides. Bull. Environ. Contam. Toxicol.5, 553 (1970).
Edwards, C. A., and A. R. Thompson: Pesticides and soil fauna. Residue Rev.45, 1 (1973).
Eisler, R.: Acute toxicities of organochlorine and organophosphorus insecticides to estuarine fishes. Tech. Paper Bur. Sport Fish. Wildl. (U.S.)46, 1 (1970).
Eto, M.: Organophosphorus pesticides: Organic and biological chemistry. Cleveland: CRC Press (1974).
Fell, J. W., R. C. Cefalu, I. M. Master, and A. S. Tallman: Microbial activities in the mangrove (Rhizophora mangle) leaf detrital system. Proc. Int. Symp. Biology and Management of Mangroves (Honolulu, Hawaii)2, 661 (1975).
Fell, J. W., I. M. Master, and S. Y. Newell: Laboratory model of the potential role of fungi in the decomposition of red mangrove (Rhizophora mangle L.) leaf litter. In K. R. Tenore and B. C. Coull (eds.): Marine benthic dynamics, p. 359. Columbia: Univ. S.C. Press (1980).
Fox, I.: Fenthion resistance inAedes aegypti from selection pressure on larvae. Mosq. News37, 452 (1977).
Froggatt, J. L.: Seasonal zooplankton diversity, abundance, and biomass across Biscayne Bay, Florida. MS Thesis. University of Miami, Coral Gables, FL (1979).
Gerlach, S. A.: Food chain relationships in subtidal silty sand marine sediments and the role of meiofauna in stimulating bacterial productivity. Oecologia (Berl.)33, 55 (1978).
Gotto, J. W., and B. F. Taylor: N2-fixation associated with decaying leaves of the red mangrove (Rhizophora mangle). Appl. Environ. Microbiol.31, 781 (1976).
Guenzi, W. D. (ed.): Pesticides in soil and water. Madison, WI: Soil Sci. Soc. Amer. (1974).
Korn, S., and R. D. Earnest: Acute toxicity of twenty insecticides to striped bass,Morone saxatilis. Calif. Fish Game60, 128 (1974).
Levy, R., and T. W. Miller: Susceptibility of the mosquito nematodeRomanomermis culcivorax (Mermithidae) to pesticides and growth regulators. Environ. Entomol.6, 447 (1977).
: Tolerance of the planarianDugesia dorotocephala to high concentrations of pesticides and growth regulators. Entomophaga23, 31 (1978).
Livingston, R. J.: Dynamics of organochlorine pesticides in estuarine systems: Effects on estuarine biota. In M. Wiley (ed.): Estuarine processes. Vol. 1. Uses, stresses, and adaptation to the estuary, p. 507. New York: Academic Press (1976).
Lugo, A. E., M. Sell, and S. C. Snedaker: Mangrove ecosystem analysis. In B. C. Patten (ed.): Systems analysis and simulation in ecology. Vol. IV, p. 113. New York: Academic Press (1976).
McLeese, D. W.: Olfactory response and fenitrothion toxicity in American lobsters (Homarus americanus). J. Fish. Res. Bd. Can.31, 1127 (1974).
Muirhead-Thomson, R. C.: Pesticides and freshwater fauna. New York: Academic Press (1971).
Newell, S. Y., R. Cefalu, and J. W. Fell:Myzocytium, Haptoglossa, andGonimochaete (fungi) in littoral marine nematodes. Bull. Mar. Sci.27, 177 (1977).
Newell, S. Y., and J. W. Fell: Mycoflora of turtlegrass (Thalassia testudinum König) as recorded after seawater incubation. Bot. Mar.23, 265 (1980).
O'Connors, H. B., C. F. Wurster, C. D. Powers, D. C. Biggs, and R. G. Rowland: Polychlorinated biphenyls may alter trophic pathways by reducing phytoplankton size and production. Science201, 737 (1978).
Odum, W. E., and E. J. Heald: The detritus-based food web of an estuarine mangrove community. In L. E. Cronin (ed.): Estuarine research. I. Chemistry, biology, and the estuarine system, p. 265. New York: Academic Press (1975).
O'Meara, G. F.: Saltmarsh mosquitoes (Diptera: Culicidae). In L. Cheng (ed.): Marine insects, p. 303. New York: American Elsevier (1976).
Parkinson, D., K. H. Domsch, and J. P. E. Anderson: Die Entwicklung mikrobieller Biomassen im organischen Horizont eines Fichtenstandortes. Oecol. Plant.13, 355 (1978).
Patterson, R. S., and D. L. Von Windeguth: The effect of Baytex on some aquatic organisms. Mosq. News24, 46 (1964).
Pimentel, D.: Ecological effects of pesticides on non-target species. Office of Science and Technology. Washington, DC: U.S. Govt. Printing Office (1971).
Provasoli, L., J. J. A. McLaughlin, and M. R. Droop: The development of artificial media for marine algae. Arch. Microbiol.25, 392 (1957).
Reeve, M. R.: Ecological significance of the Zooplankton in the shallow subtropical waters of South Florida. In L. E. Cronin (ed.): Estuarine research. I. Chemistry, biology, and the estuarine system, p. 352. New York: Academic Press (1975).
Reeve, M. R., M. A. Walter, K. Darcy, and T. Ikeda: Evaluation of potential indicators of sublethal toxic stress on marine Zooplankton (feeding, fecundity, respiration, and excretion): Controlled ecosystem pollution experiment. Bull. Mar. Sci.27, 105 (1977).
Rogers, A. J., and C. B. Rathburn: Present status of insecticides for mosquito control in Florida. Mosq. News24, 286 (1964).
Sanders, H. O., and O. B. Cope: Toxicities of several pesticides to two species of cladocerans. Trans. Amer. Fish. Soc.95, 165 (1966).
Sokal, R. R., and F. J. Rohlf: Biometry. The principles and practice of statistics in biological research. San Francisco: W. H. Freeman. (1969).
Stewart, W. E. P., G. P. Fitzgerald, and R. H. Bums:In situ studies on N2 fixation using the acetylene reduction technique. Proc. Natl. Acad. Sci. U.S.58, 2071 (1967).
Symons, P. E. K.: Dispersal and toxicology of the insecticide fenitrothion; predicting hazards of forest spraying. Residue Rev.68, 1 (1977).
Tagatz, M. E., P. W. Borthwick, G. H. Cook, and D. L. Coppage: Effects of ground applications of malathion on salt marsh environments in northwestern Florida. Mosq. News34, 309 (1974).
Tripp, M. R.: Effects of organophosphate pesticides on adult oysters (Crassostrea virginica). In F. J. Vernberg and W. B. Vernberg (eds.): Pollution and physiology of marine organisms, p. 225. New York: Academic Press (1974).
Tu, C. M.: Effect of four organophosphorus insecticides on microbial activities in soil. Appl. Microbiol.19, 479 (1970).
Wainwright, M.: A review of the effects of pesticides on microbial activity in soils. J. Soil Sci.29, 287 (1978).
Wall, W. J., and V. M. Marganian: Control ofCulicoides melleus (Coq.) (Diptera: Heleidae) with granular organophosphorus insecticides, and the direct effect on other fauna. Mosq. News31, 209 (1971).
Walsh, G. E.: Insecticides, herbicides, and polychlorinated biphenyls in estuaries. J. Wash. Acad. Sci.62, 122 (1972).
Ward, D. V., and B. L. Howes: The effects of Abate ®, an organophosphorus insecticide, on marsh fiddler crab populations. Bull. Environ. Contam. Toxicol.12, 694 (1974).
Ware, G. W., and C. C. Roan: Interaction of pesticides with aquatic microorganisms and plankton. Residue Rev.33, 15 (1970).
Weary, G. C., and H. G. Merriam: Litter decomposition in a red maple woodlot under natural conditions and under insecticide treatment. Ecology59, 180 (1978).
Zuberer, D. A., and W. S. Silver: Biological dinitrogen fixation (acetylene reduction) associated with Florida mangroves. Appl. Environ. Microbiol.35, 567 (1978).
Author information
Authors and Affiliations
Additional information
Contribution of the Rosenstiel School of Marine and Atmospheric Science, University of Miami, and the University of Georgia Marine Institute (No. 418).
Rights and permissions
About this article
Cite this article
Newell, S.Y., Cooksey, K.E., Fell, J.W. et al. Acute impact of an organophosphorus insecticide on microbes and small invertebrates of a mangrove estuary. Arch. Environ. Contam. Toxicol. 10, 427–435 (1981). https://doi.org/10.1007/BF01055439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01055439