Abstract
The following tetracyclic ring systems and their derivatives have been synthesized for pharmacological investigations: Trimethylenethieno[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-one and -4-thione (1 a, 5 a); Tetramethylenethieno[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-one and -4-thione (1 b, 1 j, 5 b); Pentamethylenethieno[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-one and-4-thione (1 c, 5 c); Trimethylenethieno[2,3—d]tetrahydropyrido[1,2—a]pyrimidin-4-one and -4-thione (1 d, 5 d); Tetramethylenethieno[2,3—d]tetrahydropyrido[1,2,—a]pyrimidin-4-one and -4-thione (1 e, 5 e); Pentamethylenethieno[2,3—d]tetrahydropyrido[1,2—a]pyrimidin-4-one and -4-thione (1 f, 5 f); Trimethylenethieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-one and -4-thione (1 g, 5 g); Tetramethylenethieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-on and -4-thione (1 h, 5 h); Pentamethylenethieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-one and -4-thione (1 i, 5 i); Tentamethylenethieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-one (7 b); Pentamethylenethieno-[2,3—d]tetrahydropyrido[1,2—a]pyrimidin-4-one (7 c).
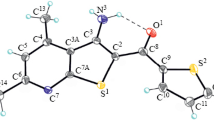
Compounds1 a–i were synthesized from 2-amino-3-ethoxycarbonyl-4,5-polymethylenethiophene2 a–c with the corresponding lactim ethers (3 a–c) in chlorobenzene in the presence of polyphosphoric acid (PPA). Compounds7 b and7 c were obtained in the reaction of β-amino acid esters2 b and2 c with 2-bromopyridine (6). The thione derivatives (5 a–i) were prepared from compounds1 a–i with phosphorus(V) sulphide.
Zusammenfassung
Für pharmakologische Untersuchungen synthetisierten wir die folgenden tetracyclischen Ringsysteme und deren Derivate: Trimethylen-thieno-[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-on und -4-thion (1 a, 5 a); Tetramethylen-thieno[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-on und 4-thion (1 b, 1 j, 5 b); Pentamethylen-thieno[2,3—d]dihydropyrrolo[1,2—a]pyrimidin-4-on und-4-thion (1 c, 5 c); Trimethylen-thieno[2,3—d]tetrahydropyrido[1,2—a]pyrimidin-4-on und -4-thion (1 d, 5 d); Tetramethylen-thieno[2,3—d]tetrahydropyrido-[1,2–a]pyrimidin-4-on und -4-thion (1 e, 5 e); Pentamethylen-thieno[2,3—d]tetrahydropyrido[1,2–a]pyrimidin-4-on und -4-thion (1 f, 5 f); Trimethylenthieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-on und -4-thion (1 g, 5 g); Tetramethylen-thieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-on und -4-thion (1 h, 5 h); Pentamethylen-thieno[2,3—d]tetrahydroazepino[1,2—a]pyrimidin-4-on und -4-thion (1 i, 5 i); Tetramethylen-thieno[2,3—d]-tetrahydropyrido[1,2—a]pyrimidin-4-on (7 b); Pentamethylen-thieno[2,3—d]-tetrahydropyrido[1,2—a]pyrimidin-4-on (7 c).
Die Verbindungen1 a–i wurden aus 2-Amino-3-(ethoxycarbonyl)-4,5-polymethylenthiophenen (2 a–c) mit den entsprechenden Lactimethern in Chlorbenzol mit Polyphosphorsäure-Katalysator dargestellt. Die Verbindungen7 b und7 c wurden aus β-Aminosäureestern2 b–c und 2-Brompyridin (6) synthetisiert. Die Thionderivate (5 a–i) erhielten wir durch die Reaktion der Verbindungen1 a–i mit Phosphor(V)-sulfid.
Similar content being viewed by others
References
Bernáth G, Fülöp F, Hermecz I, Mészáros Z, Tóth G (1979) J Heterocyclic Chem 16: 137
Hermecz I, Fülöp F, Mészáros Z, Bernáth G, Knoll J (1971) Ger Pat 2: 836,449. C.A. 91: 57048
Bernáth G,Tóth G,Fülöp F,Göndös Gy,Gera L (1979) J Chem Soc Perkin Trans 1: 1765
Fülöp F,Simon K,Tóth G,Hermecz I,Mészáros Z,Bernáth G (1982) J Chem Soc Perkin Trans 1: 2801
Manhas MS, Sharma SD (1971) J Heterocyclic Chem 8: 1051
Sauter F (1968) Monatsh Chem. 99: 2109
Patronuto de Investigacion Cientifico y Tecnics “Juan de la Gierva” and Laboratorios Mode (1973) S A Span Pat: 371, 373. CA 79: 92269b
Rosowsky A, Chaykovsky M, Chen KKN, Lin M, Modest EJ (1973) J Med Chem 16: 185
Chaykovsky M, Lin M, Rosowsky A, Modest EJ (1973) J Med Chem 16: 188
Rosowsky A, Chen KKN, Lin M (1973) J Med Chem 16: 191
Narr B, Woitun E (1973) Ger Pat 2: 200,764. CA 79: 92270v
Sauter F, Stanetty P, Schrom E (1977) Arch Pharm 310: 337
Manhas MS, Amin SG (1977) J Heterocyclic Chem 14: 161
Leistner S, Wagner G (1977) Z Chem 17: 95
Temple DL jr (1978) US Pat 4.054,656. CA 88: 37830p
Lalezari I, Jabari-Sahbari MH (1978) J Heterocyclic Chem 15: 837
Sauter F, Stanetty P, Schrom E, Sengstschmid G (1978) Monatsh Chem 109: 53
Bristol-Meyers Co (1979) Belg Pat: 859,818 (1978). CA 90: 38952h
Temple DL jr: Ger Pat 2: 746,750 (1979). CA 81: 74655 (1978)
Temple DL jr: Fr Demande 2: 401,152 (1977). CA 91: 193157 (1979)
Temple DL, Yevich JP, Covington RR, Hanning GA, Seidehamel RJ, Mackey HK, Bartek MJ (1979) J Med Chem 22: 505
Manhas MS, Amin SG, Sharma SD, Dayal D, Bose AK (1979) J Heterocyclic Chem 16: 371
Conner ST, Cetenko WA, Kerbleski JJ, Sorenson RJ, US Pat 4: 230,707. C A 94: 84162f (1980)
Süsse M, Johne S (1981) J prakt Chem 323: 647
Ishikawa F, Kosasayama A, Yamaguchi M, Watanabe Y, Saagusa J, Shibamura S, Sakuma K, Ashida S, Abiko Y (1981) J Med Chem 24: 376
Yamaguchi M, Ishikawa R (1981) J Heterocyclic Chem 18: 67
Tinney FJ, Cetenko WA, Kerbleski JJ, Connor DT, Sorenson RJ, Herzig DJ (1981) J Med Chem 24: 878
Shishoo CJ, Devani MB, Ullas GV, Ananathan S, Bhadti VS (1981) J Heterocyclic Chem 18: 43
Ram VJ, Pandey MK, Vlietinek AJ (1981) J Heterocyclic Chem 18: 1277
Haubold G, Pech R, Bernáth G, Lázár J, Csukonyi K, Hirschelmann R, Laban G, Böhm R (1983) Pharmazie 38: 269
Perrissin M, Favre M, Duc C L, Bakri-Logeais F, Huguet F, Narcisse G (1984) Eur J Med Chem 19: 420
Gewald K (1961) Angew Chem 73: 114
Gewald K (1962) Z Chem 2: 40
Gewald K, Schinke S, Böttcher H (1966) Chem Ber 90: 94
Ramanthan JD, Nauboothiri DG, Shah GF, Mahta HJ, Padhya AG (1978) J Indian Chem Soc 58: 922
Perrissin M, Duc CL, Narcisse G, Bakri-Logeais F, Huguet F (1980) Eur J Med Chem 15: 413
Perrissin M, Duc CL, Narcisse G, Bakri-Logeais F, Huguet F (1980) Eur J Med Chem 15: 563
Gauthier R, Blandeau P, Berse G, Gravel D (1981) Can J Chem 48: 2612
Wamhoff H, Lichtenthaler L (1978) Chem Ber 111: 2297
Legrand L, Lozac'h M (1967) Bull Soc Chim France: 2067
Legrand L (1960) Bull Soc Chim France: 337
Baker W, Harborne JB, Ollis WD (1952) J Chem Soc: 1303
Dash B, Dora EK, Panda CS (1982) J Heterocyclic Chem 19: 2093
Simonis H, Rosenberg S (1914) Berichte 47: 1232
Antaki H, Petrov V (1951) J Chem Soc: 551
Author information
Authors and Affiliations
Additional information
Part 74:Szabó J, Fodor L, Szűcs E, Bernáth G, Sohár P (1984) Pharmazie 39: 347.
Rights and permissions
About this article
Cite this article
Csukonyi, K., Lázár, J., Bernáth, G. et al. Saturated heterocycles, 75. Preparation of tetracyclic thiophene derivatives with bridgehead nitrogen. Synthesis of polymethylenethieno[2,3—d]dihydropyrrolo-, tetrahydropyrido- and tetrahydroazepino[1,2—a]pyrimidin-4-ones and -4-thiones. Monatsh Chem 117, 1295–1303 (1986). https://doi.org/10.1007/BF00810875
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00810875