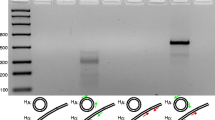
Summary
Romanomennis culicivorax, an obligate parasitic nematode of mosquitos, possesses an unusually large mitochondrial genome. Individuals are monomorphic for one of several mitochondrial DNA (mtDNA) size variants ranging from 26–32 kb. In this report, we demonstrate that the mitochondrial genome size differential in three isofemale lineages is due to the presence of mtDNA sequences amplified to different copy numbers within each mtDNA molecule. Restriction enzyme analysis and DNA sequencing studies reveal that each mitochondrial genome contains one of two 3.0 kb repeat types that differ by approximately 30 bp. This difference is primarily due to a short (23 bp) imperfect tandem duplication present within the larger of two polymorphic repeating units. The 3.0 kb reiterated DNA sequences are present as direct, tandem repeats and as inverted portions of the same sequence located elsewhere in the genome. Based on mtDNA analysis of an independently reared R. culicivorax culture, we conclude that events resulting in mitochondrial genome rearrangement occurred in natural field populations prior to propagation within the laboratory.
Similar content being viewed by others
References
Attardi G (1985) Int Rev Cytol 93:93–145
Bentzen P, Leggett WC, Brown GG (1988) Genetics 118:509–518
Bolivar F (1978) Gene 4:121–136
Brown WM (1985) The mitochondrial genome of animals. In: MacIntyre RJ (ed) Molecular evolutionary genetics. Plenum Press, New York, pp 95–130
Clary DO, Wolstenholme DR (1985) J Mol Evol 22:252–271
Henikoff S (1984) Gene 28:351–359
Hyman BC, Beck JL, Weiss KC (1988) Genetics 120:707–712
Kessler LG, Avise JC (1985) Mol Biol Evol 2:109–125
LePecq J-B, Paoletti C (1967) J Mol Biol 27:87–106
Levinson G, Gutman GA (1987) Mol Biol Evol 4:203–221
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor NY
Moritz C, Brown WM (1986) Science 233:1425–1427
Moritz C, Brown WM (1987) Proc Natl Acad Sci USA 84:7183–7187
Petersen JJ, Chapman HC, Woodard DB (1968) Mosq News 28:346–352
Powers TO, Platzer EG, Hyman BC (1986a) Curr Genet 11:71–77
Powers TO, Platzer EG, Hyman BC (1986b) J Nematol 18:288–293
Rigby PW, Dieckmann M, Rhodes C, Berg P (1977) J Mol Biol 113:237–251
Sanger F, Nicklen S, Coulson AR (1977) Proc Natl Acad Sci USA 74:5463–5467
Sederoff RR (1984) Structural variation in mitochondrial DNA. In: Scandalios JG, Caspari EW (eds) Advances in genetics, vol 22. Academic Press, New York London, pp 1–108
Snyder M, Fraser AR, LaRoche J, Gartner-Kepkay KE, Zouros E (1987) Proc Natl Acad Sci USA 84:7595–7599
Southern EM (1975) J Mol Biol 98:503–517
Stark GR, Wahl GM (1984) Annu Rev Biochem 53:447–491
Vieira J, Messing J (1987) Methods Enzymol 153:3–11
Wallis GP (1987) Heredity 58:229–238
Waring MJ (1965) J Mol Biol 13:269–282
Wolstenholme DR, Clary DO, MacFarlane JL, Wahleithner JA, Wilcox L (1985) Organization and evolution of invertebrate mitochondrial genomes. In: Quagliariello E, Slater EC, Palmieri F, Saccone C, Kroon AM (eds) Achievements and perspectives of mitochondrial research, vol II: Biogenesis. Elsevier, Amsterdam New York, pp 61–69
Wolstenholme DR, MacFarlane JL, Okimoto R, Clary DO, Wahleithner JA (1987) Proc Natl Acad Sci USA 84:1324–1328
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beck, J.L., Hyman, B.C. Role of sequence amplification in the generation of nematode mitochondrial DNA polymorphism. Curr Genet 14, 627–636 (1988). https://doi.org/10.1007/BF00434089
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00434089