Abstract
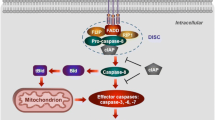
Since mammalian cardiac myocytes essentially rely on aerobic energy metabolism, it has been assumed that cardiocytes die in a catastrophic breakdown of cellular homeostasis (i.e. necrosis), if oxygen supply remains below a critical limit. Recent observations, however, indicate that a process of gene-directed cellular suicide (i.e. apoptosis) is activated in terminally differentiated cardiocytes of the adult mammalian heart by ischemia and reperfusion, and by cardiac overload as well. Apoptosis or programmed cell death is an actively regulated process of cellular self destruction, which requires energy and de novo gene expression, and which is directed by an inborn genetic program. The final result of this program is the fragmentation of nuclear DNA into typical “nucleosomal ladders”, while the functional integrity of the cell membrane and of other cellular organelles is still maintained. The critical step in this regulated apoptotic DNA fragmentation is the proteolytic inactivation of poly-[ADPribose]-polymerase (PARP) by a group of cysteine proteases with some structural homologies to interleukin-1β-converting enzyme (ICE-related proteases [IRPs] such as apopain, yama and others). PARP catalyzes the ADP-ribosylation of nuclear proteins at the sites of spontaneous DNA strand breaks and thereby facilitates the repair of this DNA damage. IRP-mediated destruction of PARP, the ‘supervisor of the genome’, can be induced by activation of membrane receptors (e.g. FAS or APOI) and other signals, and is inhibited by activation of ‘anti-death genes’ (e.g. bcl-2). Overload-triggered myocyte apoptosis appears to contribute to the transition to cardiac failure, which can be prevented by therapeutic hemodynamic unloading. In myocardial ischemia, the activation of the apoptotic program in cardiocytes does not exclude their final destiny to catastrophic necrosis with release of cytosolic enzymes, but might be considered as an adaptive process in hypoperfused ventricular zones, sacrificing some jeopardized myocytes to regulated apoptosis, which may by less arrhythmogenic than necrosis with the primary disturbance of membrane function.
Similar content being viewed by others
References
Katz AD: Physiology of the heart. Raven Press, New-York, First Edition, 1977, 422–423
Katz AD: Physiology of the heart. Raven Press, New-York, Sec. Edition, 1992, 632–635
James TNMD: Normal and abnormal consequences of apoptosis in the human heart from postnatal morphogenesis to paroxysmal arrhythmias. Circulation 90: 556–573, 1994
Takeda K, Yu ZX, Nishikawa T, Tanaka M, Ando A, Ferrans VJ, Takeshi K: Immunohistochemical study of apoptosis in the bulbus cordis of the developing rat heart (Abstr). Circulation 92: 1–306, 1995
Kajstura J, Mansukhani M, Cheng W, Reiss K, Krajewski S, Reed JC, Quaini F, Sonnenblick EH, Anversa P: Programmed cell death and expression of the protooncogene bcl-2 in myocytes during postnatal maturation of the heart. Exp Cell Res 219: 110–121, 1995
Kajstura J, Cheng W, Reiss K, Sonnenblick EH, Olivetti G, Anversa P: Apoptotic and necrotic myocyte cell death are independent contributing variables of infarct size in rats. Circulation 92: 1–772, 1995
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL: Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94: 1621–1628, 1994
Hotta K, Nakai K: Is cell death of rat cardiomyocytes following transient ischemia apoptosis? Circulation 92: 1–772, 1995
Suzuki H, Wildhirt SM, Dudek RR, Narayan KS, Bailey AH, Bing RJ: Apoptosis of macrophages in myocardial infarction. Circulation 92: 1773, 1995
Shirakawa K, Miura T, Yamakawa K, Kawamura S, Tatsuno H, Fujii A, Nakamura Y, Ryoke T, Matsuzaki M: Effects of ischemic preconditioning on the expression of TNF-α and the induction of apoptosis in the ischemia-reperfused rat heart. Circulation 92: 1–773, 1995
Elsässer A, Müller KD, Strasser R, Vogt AM, Rau M, Klövekorn WP, Schaper J: ‘Hibernating myocardium’: Degeneration caused by apoptosis. Circulation 92: I-186, 1995
Sawa Y, Bai HZ, Suzuki K, Tsujimoto Y, Matsuda H: Overexpression of bel-2 gene improves the myocardial tolerance to ischemia-reperfusion by preventing DNA fragmentation. Circulation 92: I-772, 1995
Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M: Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res 75: 426–433, 1994
Long X, Boluyt MO, Cirielli C, Capogrossi MC, Lakatta EG, Crow MT: Enhanced expression of p53 in hypoxia-induced apoptosis of cultured neonatal rat cardiomyocytes. Circulation 92: I-772, 1995
Umansky SR, Cuenco GM, Khutzian SS, Barr PJ, Tomei LD: Postischemic apoptotic death of rat neonatal cardiomyocytes. Cell Death Diff 9: 235–241, 1995
Kaqisaki K, Ichikawa H, Shirakura R, Matsuda H: Hypoxia induces DNA damage in adult cardiomyocytes: evidence for hypoxia induced apoptosis. Circulation 92: I-772, 1995
Sonnenblick EH, Cheng W, Li B, Kajstura J, Li P, Wolin MS, Olivetti G, Anversa P: Load dependent-induced programmed myocyte cell death. Circulation 99: I-567, 1995
Harriet P, Richard L, Dam TV, Teiger E, Orlov SN, Gaboury L, Gossard F, Tremblay J: Apoptosis in target organs of hypertension. Hypertension 26: 642–648, 1995
Bing OHL: Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol 26:943–948, 1994
Li Z, Lakatta EG, Robinson KG, Bing OHL: Detection of apoptosis in the failing heart of spontaneously hypertensive rats. Circulation 92: I-526, 1995
Sharov VG, Sabbah HN, Goussev AV, Lesch M, Goldstein S: Increased expression of apoptosis associated p-53 protein in cardiocytes of dogs with chronic heart failure. Circulation 92: I-525, 1995
Haider N, Narula J, Hajjar RJ, Salvo TD, Semigran MJ, Dee GW, Khaw BA: Apoptosis in human explanted cardiomyopahy hearts suggests programmed progression of dilated cardiomyopathy. Circulation 92: I-724, 1995
Olivetti G, Liu Y, Cigola E, Cheng W, Kajastura J, Hintze TH, Anversa P: Apoptosis and myocyte regeneration in ventricular pacing-induced cardiomyopathy. Circulation 92:I-526, 1995
Michler RE, Szabolcs M, Roy D, Aji W, Yang Y, Yang X. Sciacca RR, Cannon PJ: Apoptosis of cardiac myocytes parallels the induction of nitric oxide synthase (iNOS) during cardiac allograft rejection. Circulation 92: I-123, 1995
Pinsky DJ, Yang Y, Aji W, Szabolcs M, Liao H, Sciacca RR, Cannon PJ: Nitric oxide induces apoptosis of adult rat cardiac myocytes. Circulation 92: I-565, 1995
Isaacs JT: Role of programmed cell death in carcinogenesis. Environ Health Perspect 101, Suppl. 5: 27–34, 1993
Falcieri E, Gobbi P, Zamai L, Vitale M: Ultrastructural features of apoptosis. Scanning Microsc 8: 653–666, 1994
Buttke TM, Sandstrom PA: Oxidative stress as a mediator of apoptosis. Immunology Today 15: 7–10, 1994
Bright J, Khar A: Apoptosis: Programmed cell death in health and disease. Biosci Reports 14: 67–82, 1994
Trump BF, Berezesky IK: Calcium-mediated cell injury and cell death. FASEB J 9: 219–228, 1995
Kerr JFR, Wyllie AH, Currie AR: Apoptosis: A basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer 26:239–257, 1972
Clarke PG, Clarke S: Historic apoptosis. Nature 378: 230–230, 1995
Peitsch MC, Mannherz HG, Tschopp J: The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol 4: 37–41, 1994
Walker PR, Sikorska M: Endonuclease activities, chromatin structure, and DNA degradation in apoptosis. Biochem Cell Biol 72: 615–623, 1994
Kerr JFR, Winterford CM, Harmon BV: Apoptosis: Its significance in cancer and cancer therapy. Cancer 73: 2013–2026, 1994
Columbano A: Cell death: Current difficulties in discriminating apoptosis from necrosis in the context of pathological processes in vivo. J Cellular Biochem 58: 181–190, 1995
Majno G, Joris I: Apoptosis, oncosis and necrosis — An overview of cell death. Am J Pathol 146: 3–15, 1995
Duvall E, Wyllie AH: Death and the cell. Immunology Today 7: 115–119, 1986
Ferrer I, Martin F, Reiriz J, Perez-Navarro E, Albrech J, Macaya A, Planas AM: Both apoptosis and necrosis occur following intrastriatal administration of exocytotoxins. Acta Neuropathol (Berl.) 90: 504–510, 1995
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961–973, 1995
Popper H: Hepatocellular degeneration and death. In: IM Aeias, WB Jakoby, H Popper, D Schachter, DA Shafritz (eds). The Liver: Biology and Pathobiologie Second. Edit. Raven Press, Ltd, New York, 1988, pp 1087–1103
Schwartz LM, Osborne BA: Programmed cell death, apoptosis and killer genes. Immunology Today 14: 582–590, 1993
Hengartner MO, Horvitz HR: The ins and outs of programmed cell death during C. elegans development. Philos Trans R Soc Lord (Biol.) 345: 243 -246, 1991
Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR: The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75: 641–652, 1993
Yuan J, Horvitz HR: The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during period of extensive programmed cell death. Development 116: 309–320, 1992
Hengartner MO, Ellis RE, Horvitz HR: Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356: 494–499, 1992
Nagata S: Fas and Fas ligand: A death factor and its receptor. Advances in Immunology 57: 129–144, 1994
Nicholson DW, Ali A, Thomberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffith PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL, Miller DK: Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43, 1995
Cheng L, Liu C, Koopman WJ, Mountz JD: Characterization of human fas-gene exon/intron organization and promotor region. J Immunology 154: 1239–1245, 1995
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T: p53 is required for radiation induced apoptosis in mouse thymocytes. Nature 362: 847–849, 1993
Dieken ES, Miesfeld RL: Transcriptional transactivation functions localized to the glucocorticoid receptor N-terminus are necessary for steroid induction of lymphocyte apoptosis. Mol Cell Biol 12: 589–597, 1992
Chittenden T, Harrington EA, O'Connor R, Flemington C, Lutz RJ, Evan GI, Guild BC: Induction of apoptosis by the Bcl-2 homologue Bak. Nature 374 733–736, 1995
Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ: Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 374: 736–739, 1995
Bargou RC, Bommert K, Weinmann P, Daniel PT, Wagener C, Mapara MY, Dörken B: Induction of Bax-α precedes apoptosis in a human B lymphoma cell line. Eur J Immunol 25: 770–775, 1995
Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ: Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334–336, 1990
Apte SS, Mattei MG, Seldin MF, Olson BR: The highly conserved defender against the death 1 (DAD 1) gene maps to human chromosome 11q11-q 12 and mouse chromosome 14 and has plant and nematode homologs. FEBS Lett 363: 304–306, 1995
Sugimoto A, Hozak RR, Nakashima T, Nishimoto T, Rothman JH: Dad-1 an endogenous programmed cell death suppressor in Caenorhabditis elegans and vertebrates. EMBO J 14: 4434–4441, 1995
Nakashima T, Sekiguchi T, Kuraoka A, Fukushima K, Shibata Y, Komiyama S, Nishimoto T: Molecular cloning of a human cDNA encoding a novel protein, DAD-1, whose defect causes apoptotic cell death in hamster BHK21 cells. Molec Cell Biol 13: 6367–6374, 1993
Ylug IG, See CG, Fisher EMC: The DAD-1 protein, whose defect causes apoptotic cell death, maps to human chromosome 14. Genomics 26: 433–435, 1995
Tewari M, Dixit VM: Fas- and Tumor Necrosis Factor-induced Apoptosis is inhibited by the Poxvirus crmA Gene Product. J Biol Chem 270: 3255–3260, 1995
Tewari M, Beidler DR, Dixit VM: CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem 270: 18738–18741, 1995
Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM: Yama/CPP32β, a mammalian homolog of Ced-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81: 801–809, 1995
Beidler DR, Tewari M, Friesen PD, Poirier G, Dixit VM: The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem 270: 16526–16528, 1995
Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi L, Greenber AH, Miller LK, Wong WW: Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 269: 1885–1888, 1995
Schulze-Osthoff K: The Fas/APO-1 receptor and its deadly ligand. Trends Cell Biol 4: 421–426, 1994
Behrmann I, Walczak H, Krammer PH: Structure of the human APO1-gene. Eur J Immunol 24: 3057–3062, 1994
Nagata S: Apoptosis regulated by a death factor and its receptor: Fas ligand and Fas. Phil Trans R Soc London B 345: 281–287, 1994
Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, Nagata S: Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp Cell Res 215: 332–337, 1994
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD: Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 261: 1759–1762, 1994
Chinnnaiyan AM, O'Rourke K, Tewari M, Dixit VM: FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81: 505–512, 1995
Itch N, Nagata S: A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem 268: 10932–10937, 1993
Los M, Van de Craen M, Penning LC, Schenk H, Westendorp M, Baeuerle PA, Dröge W, Krammer PH, Fiers W, Schulze-Osthoff K: Requirement of an ICE/CED-3 protease for Fas/APO-1 mediated apoptosis. Nature 375: 81–83, 1995
Tanaka M, Suda T, Takahashi T, Nagata S: Expression of the functional soluble form of the human Fas ligand in activated lymphocytes. EMBO J 14: 1129–1135, 1995
Suda T, Nagata S: Purification and characterization of the Fas ligand that induces apoptosis. J Exp Med 179: 873–879, 1994
Aggarwal BB, Singh S, LaPushin R, Totpal K: Fas antigen signals proliferation of normal human diploid fibroblasts and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 364: 5–8, 1995
Peitsch MC, Tschopp J: Comparative molecular modelling of the Fas ligand and other members of the TNF family. Mol Immunol 32: 761–765, 1995
McClonskey TW, Oyaizu N, Kaplan M, Pahwa S: Expression of the Fas antigen in patients infected with human immundeficiency virus. Cytometry 22: 111–114, 1995
Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H: Metalloproteinase-mediated release of human Fas-ligand. J Exp Med 182: 1777–1783, 1995
Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A: Protooncogene bel-2 transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradation. J Clin Invest 95: 2633–2643, 1995
Whyte M, Evan G: The last cut is the deepest. Nature 376: 17–18, 1995
Nunez G, Clarke MF: The Bcl-2-family of proteins: regulators of cell death and survival. Trends Cell Biol 4: 399–403, 1994
Panayiotidis P, Ganeshaguru K, Foroni L, Hoffbrand AV: Expression and function of the Fas antigen in B chronic lymphocytec leukemia and hairly cell leukemia. Leukemia 9: 127–1232, 1995
Shinohara S, Sawada T, Nishioka Y, Tolima S, Kisaki T, Inoue T, Ando K, Ikeda M, Fujii H, Ito K: Differential expression of Fas antigen and Bcl-2 protein on CD4+ T cells. CD8+ T cells, and monocytes. Cell Immunol 163: 303–308, 1995
Massaia M, Borrione P, Attisano C, Barral P, Beggiato E, Montacchini L, Bianchi A, Boccadoro M, Pileri A: Dysregulated Fas and Bcl-2 expression leading to enhanced apoptosis in T cells of multiple myeloma patients. Blood 85: 3679–3687, 1995
Lu PJ, Lu QL, Rughetti A, Taylor-Papadimitriou J: Bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorgenesis of human mammary epithelial cells. J Cell Biol 129: 1363–1378, 1995
Hockenbery DM: Bcl-2, a novel regulator of cell death. BioEssays 17: 631–638, 1995
Riparbelli MG, Callaini G, Tripodi SA, Cintorino M, Tosi O, Dallai R: Localization of the Bcl-2 protein to the outer mitochondrial membrane by electrone microscopy. Exp Cell Res 221: 363–369, 1995
Nguyen M, Branton PE, Waltons PA, Oltvai ZN, Korshmeyer SJ, Shore GC: Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. J Biol Chem 269: 16521–16524, 1994
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ: Bcl-2 function in an antioxidant pathway to prevent apoptosis. Cell 75: 241–251, 1993
Steinman HM: The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem 270: 3487–3490, 1995
Giordano C, Stassi G, Todaro M, De Maria R, Richiusa P, Scorsone A, Giordano M, Galluzo A: Low bcl-2 expression and increased spontaneous apoptosis in T-lymphocytes from newly-diagnosed IDDM patients. Diabetologia 38: 953–958, 1995
Armant M, Delespesse G, Sarfati M: IL-2 and IL-7 but not IL-12 protect natural killer cells from death by apoptosis and up-regulate bcl-2 expression. Immunology 85: 331–337, 1995
Tamura A, Yui K: Age-dependent reduction of Bcl-2 expression in peripheral T cells of lpr and gid mutant mice. J Immunol 155: 499–507, 1995
Bruel A, Benoit G, DeNay D, Brown S, Lanotte M: Distinct apoptotic responses in maturation sensitive and resistant t(15; 17) acute promyelocytic leukemia NB4 cells. 9-cis retinoic acid induces apoptosis independent of maturation and Bcl-2 expression. Leukemia 9: 1173–1184, 1995
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ: Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA 92:7834–7838, 1995
Kumar S: ICE-like proteases in apoptosis. Trends Biol Sci 20: 198–202, 1995
Vasilakov JP, Ghayur T, Carroll RT, Giegel DA, Saunders JM, Quintal L, Keane KM, Shivers BD: IL- I β converting enzyme (ICE) is not required for apoptosis induced by lymphokine deprivation in IL-2 dependent T cell line. J Immunology 155: 3433–3442, 1995
Kumar S: Inhibition of apoptosis by the expression of antisense Nedd2. FEBS Lett 368: 69–72, 1995
Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA: Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1β-converting enzyme. Genes Dev 8: 1613–1326, 1994
Fernandes-Alnemri T, Litwack G, Alnemri ES: Mch2 a new member of the apoptotic Ced-3/ICE cysteine protease gene family. Cancer Res 55: 2737–2742, 1995
Munday NA, Vaillancourt JP, Ali A, Casano FJ, Miller DK, Molineaux SM, Yamin TT, Yu VL, Nicholson DW: Molecular cloning and proapoptotic activity of ICEreII and ICEreIII, members of the ICE/Ced 3 family of cysteine proteases. J Biol Chem 270: 15870–15876, 1995
Fernandes-Alnemri T, Tajahashi A, Armstrong R, Krebs J, Fritz L, Tomaselli KJ, Wang L, Yu Z, Croce CM, Salveson G, Earnshaw WC, Litwack G, Alnemri ES: Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res 55: 6045–6052, 1995
Lazebnik YA, Kaufmann SH, Desneuers S, Poirier GG, Eamshaw WC: Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347, 1994
Martin, SJ, Green DR: Protease activation during apoptosis: Death by a thousand cuts? Cell 82: 349–352, 1995
Schlegel J, Peters I, Orrenius S: Isolation and partial characterization of a protease involved in Fas-induced apoptosis. FEBS Lett 364: 139–142, 1995
Kwo P, Patel T, Bronk SF, Gores GJ: Nuclear serine protease activity contributes to bile acid-induced apoptosis in hepatocytes. Am J Physiol 268: G613-G621, 1995
Zhivotovsky B, Wade D, Gahm A, Orrenius S, Nicotera P: Formation of 50 kbp chromatin fragments in isolated nuclei is mediated by protease and endonuclease activation. FEBS Lett 351: 150–154, 1994
Darmon AJ, Nicholson DW, Bleackley RC: Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 377: 446–448, 1995
Enari M, Hug H, Nagata S: Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature 375: 78–81, 1995
Smyt MJ, Trapani JA: Granzymes: Exogenous proteinases that induce target cell apoptosis. Immunology Today 16: 202–206, 1995
Brancolini C, Benedetti M, Schneider C: Microfilament reorganization during apoptosis. The role of Gas2, a possible substrate for ICElike proteases. EMBO J 14: 5179–5190, 1995
Mashima T, Naito M, Fujita N, Noguchi K, Tsuruo T: Identification of actin as a substrate of ICE and ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun 217: 1185–1192, 1995
Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamn R, Weichselbaum R, Kufe D: Proteolytic activation of proteinkinase CS by an ICE-like protease in apoptotic cells. EMBO J 14: 6148–6156, 1995
Larrick JW, Wright SC: Cytotoxic mechanism of tumor necrosis factor-α. FASEB J 4: 3215–3223, 1990
De Murcia G, De Murcia JM: Poly(ADP-ribose) polymerase: A molecular nick-sensor. Trends Biochem Sci 19: 172–176, 1994
Masutani M, Nozaki T, Wakabayashi K, Sugimura T: Role of poly(ADP-ribose) polymerase in cell-cycle checkpoint mechanism following gamma-irradiation. Biochemie 77: 462–465, 1995
Sugimura T, Miwa M: Poly(ADP-ribose): Historical perspective. Mol Cell Biochem 138: 5–12, 1994
Althaus FR, Mathis G: ADP-Ribosylation. DNA Repair, and Chromatin organization. In: F.R. Althaus, H. Hilz, S. Shall S (eds). ADP-Ribosylation of proteins. Springer Verlag Berlin 1985 pp 235–241
Yoshihara K, Itaya A, Tanaka Y, Ohashi Y, Ito K, Teraoka Y, Tsukada K, Matsukage A, Kamiya T: Poly(ADP-ribos)ylation of nuclear enzymes. In: F.R. Althaus, H. Hilz, S. Shall (eds). ADP-Ribosylation of proteins. Springer Verlag Berlin 1985, pp 82–92
Creissen D, Shall S: Regulation of DNA ligase activity by poly(ADP-ribose). Nature 266: 266–271, 1992
Giannoni P, Scarabelli L, Orunesu M, Cesarone CF: In vitro effect of 3,5,3,′Triiodothyronine on poly(ADP-Ribosyl)ation of DNA Topoisomerase I. Italian J Biochemistry 44: 129–136, 1995
Althaus FR, Bachmann S, Hofferer L, Kleczkowska HE, Malanga M, Panzeter PL, Realini C, Zweifel B: Interactions of poly(ADP-ribose) with nuclear proteins. Biochimie 77: 423–432, 1995
Althaus FR, Hofferer L, Kleczkowska HE, Malanga M, Naegeli H, Panzeter PL, Realini CA: Histone shuttling by poly ADP-ribosylation. Mol Cell Biochem 138: 53–59, 1994
Alvarez-Gonzalez R, Mendoza-Alvarez H: Dissection of ADP-ribose polymer synthesis into individual steps of initiation, elongation, and branching. Biochimie 77: 403–407, 1995
Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis P, Shall S, Ziaee AA: The involvement of poly-(ADP-ribose) polymerase in the degradation of NAD+ caused by gamma irradiation and N-methyl-N-nitrosourea. Eur J Biochem 101: 135–145, 1979
Küpper JH, Van Gool L, Bürkle A: Molecular genetic system to study the role of poly-(ADP-ribosyl) action in the cellular responce to DNA damage. Biochimie 77: 450–455, 1995
Desneuers S, Shah GM, Brochu G, Hoflack JC, Verreault A, Poirier GG: Biochemical properties and function of poly-(ADP-ribose) glycohydrolase. Biochimie 77: 433–438, 1995
Rice WG, Hillyer CD, Harten B, Schaeffer CA, Dorminy M, Lackey III DA, Kirsten E, Mendeleyev J, Buki KG, Hakam A, Kun E: Induction of endonuclease-mediated apoptosis in tumor cells by C nitroso-substituted ligands of poly(ADP-ribose)polymerase. Proc Nat Acad Sci USA 89: 7703–7707, 1992
Bortner CD, Oldenburg NBE, Cidlowski JA: The role of DNA fragmentation in apoptosis. Trends Cell Biol 5: 21–26, 1995
Walker PR, Sikorska M: Endonuclease activities, chromatin structure, and DNA degradation in apoptosis. Biochem Cell Biol 72: 615–623, 1994
Ueda N, Walker PD, Hsu SM, Shah SV: Activation of a 15 kDa endonuclease in hypoxia/reoxygenation injury without morphologic features of apoptosis. Proc Natl Acad Sci USA 92: 7202–7206, 1995
Russo CA, Weber TK, Volpe CM, Stoler DL, Petrelli NJ, Rodriguez-Bigas M, Burhans WC, Anderson GR: An anoxia inducible endonuclease and enhanced DNA breakage as contributors to genomic instability in cancer. Cancer Res 55: 1122–1128, 1995
Heron A, Pollard H, Dessi F, Moreau J, Lasbennes F, Ben Ari Y, Charriaut-Marlangue C: Regional variability in DNA-fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem 61: 1973–1976, 1993
Duke RC, Witter RZ, Nash PB, Young JDE, Ojcius DM: Cytolysis mediated by ionophores and pore-forming agents: Role of intracellular calcium in apoptosis. FASEB J 8: 237–246, 1994
Ferrer I, Macaya A. Blanco R, Olive M, Cinos C, Munell F, Planas AM: Evidence of intemucleosomal DNA fragmentation and identification of dying cells in X-ray-induced cell death in developing brain. Int J Neurosci 13: 21–28, 1995
Elia MC, Stoler RD, McKelvey TW, Kraynak AR, Barnum JE, Harmon LS, DeLuca JG, Nichols WW: Rapid DNA degradation in primary rat hepatocytes treated with diverse cytotoxic chemicals. Environ Mol Mutagen 24: 181–191, 1994
Panday S, Walker PR, Sikorska M: Separate pools of endonuclease activity are responsible for internucleosomal and high molecular mass DNA fragmentation during apoptosis. Biochem Cell Biol 72: 625–629, 1994
Walker PR, Weaver VM, Lach B, Le Blanc J, Sikorska M: Endonuclease activities associated with high molecular weight and internucleosomal DNA fragmentation in apoptosis. Exp Cell Res 213: 100–106, 1994
Zhivotovsky B, Nicotera P, Bellomo F, Hanson K, Orrenius S: Ca2+ and endonuclease activation in radiation-induced lymphoid cell death. Exp Cell Res 207: 163–170, 1993
Meireles Ribeiro J, Carson DA: Ca 2+/Mg2+-dependent endonuclease from human spleen: Purification, properties, and role in apoptosis. Biochemistry 32: 9129–9136, 1993
Takauji R, Yoshida A, Iwasaki H, Tohyama K, Ueda T, Nakamura T: Enhancement of Ca2+-dependent endonuclease activity in L1210 cells during apoptosis induced by 1-β-D-arabinofuranosylcytosine: Possible involvement of activating factor(s). Jpn J Cancer Res 86: 677–684, 1995
Yanagisawa-Shiota F, Sakagami H, Kuribayashi N, Iida M, Sakagami T, Takeda M: Endonuclease activity and induction of DNA fragmentation in human myelogenous leukemic cell lines. Anticancer Res 15: 259–266, 1995
Sokolova IA, Cowan KH, Schneider E: Ca2−/Mg2+-dependent endonuclease activation is an early event on VP-16-induced apoptosis of human breast cancer MCF7 cells in vitro. Biochim Biophys Acta Mol Cell Res 1266: 135–142, 1995
Compton MM: Programmed cell death in avian thymocytes: Role of the apoptotic endonucleases. Poult Sci 72: 1267–1272, 1993
Shemtov MM, Cheng DLW, Kong L, Shu WP, Sassaroli M, Droller MJ, Liu BCS: LAK cell mediated apoptosis of human bladder cancer cells involves a pH-dependent endonuclease system in the cancer cell: Possible mechanism of BCG therapy. J Urol 154: 269–274, 1995
Fernandes RS, Cotter TG: Activation of a calcium magnesium independent endonuclease in human leukemic cell apoptosis. Anticancer Res 13: 1253–1260, 1993
Matsubara K, Kubota M, Kuwakado K, Hirota H, Wakazono Y, Okuda A, Bessho R, Lin YW, Adachi S, Akiyama Y: Variable susceptibility to apoptosis induced by calcium ionophore in hybridomas between HL-60 promyelocytic and CEM T-lymphoblastic leukemia cell lines: Relationship to constitutive Mg2+-dependent endonuclease. Exp Cell Res 213: 412–417, 1994
Perez-Sala D, Collado-Escobar D, Mollinedo F: Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J Biol Chem 270: 6235–6242, 1995
De Murcia G, Niedergang C, Bürkle A: ADP-ribosylation reactions: Mechanism and biological significance. Part 1. Introduction. Biochimie 77: 311–311, 1995
Gjertsen BT. Doskeland SO: Protein phosphorylation in apoptosis. Biochim Biophys Acta 1269: 187–199, 1995