Abstract
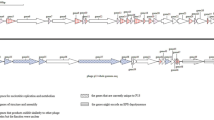
Carboxypeptidase Z is a serine carboxypeptidase secreted by Absidia zychae NRIC 1199. The cDNA and genomic DNA carrying the scpZ gene encoding carboxypeptidase Z were cloned and sequenced. The nucleotide sequences of the cDNA (1.4 kb) and the genomic DNA (3.3 kb) were analyzed and the intervening sequences were located by a comparison of the two. It was found that the scpZ gene was interrupted by 11 short introns, 50–75 nucleotides in length. Genomic Southern analysis showed that there was only one scpZ gene in the genome of A. zychae. The gene encoded a putative pre-proenzyme composed of 409 amino-acid residues of the mature carboxypeptidase Z (Mr 45 421) and an additional N-terminal sequence of 51 amino-acid residues. The amino-acid sequence around the active serine residue of carboxypeptidase Z (-G-E-S-Y-G-G-) differed from the consensus (-G-E-S-Y-A-G-) which is conserved in most of the serine carboxypeptidases so far analyzed.
Similar content being viewed by others
References
Ashikari T, Nakamura N, Tanaka Y, Kiuchi N, Shibano Y, Tanabe T, Amachi T, Yoshizumi H (1986) Rhizopus raw-starch-degrading glucoamylase: its cloning and expression in yeast. Agric Biol Chem 50:957–964
Bai Y, Hayashi R (1979) Properties of the single sulfhydryl group of carboxypeptidase Y. Effects of alkyl and aromatic mercurials on activities toward various synthetic substrates. J Biol Chem 254:8473–8479
Baulcombe DC, Barker RF, Jarvis MG (1987) A gibberellin-responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem 262:13726–13735
Bech LM, Breddam K (1989) Inactivation of carboxypeptidase Y by mutational removal of the putative essential histidyl residue. Carlsberg Res Commun 54:165–171
Breddam K (1983) Modification of the single sulfhydryl group of carboxypeptidase Y with mercurial influence on enzyme specificity. Carlsberg Res Commun 48:9–19
Breddam K (1984) Chemically modified carboxypeptidase Y with increased amidase activity. Carlsberg Res Commun 49:535–554
Breddam K (1986) Serine carboxypeptidases. A review. Carlsberg Res Commun 51:83–128
Breddam K, Sørensen SB, Svendsen I (1987) Primary structure and enzymatic properties of carboxypeptidase II from wheat bran. Carlsberg Res Commun 52:297–311
Burmester A, Wöstemeyer A, Wöstemeyer J (1990) Integrative transformation of a zygomycete, Absidia glauca, with vectors containing repetitive DNA. Curr Genet 17:155–161
Doan NP, Fincher GB (1988) The A- and B- chains of carboxypeptidase I from germinated barley originate from a single precursor polypeptide. J Biol Chem 263:11106–11110
Friedman M, Krull H, Cavins JF (1970) The chromatographic determination of cystine and cystein residues in proteins as S-β (4-pyridylethyl) cystein. J Biol Chem 254:3868–3871
Hayashi R, Moore S, Stein WH (1973) Serine at the active center of yeast carboxypeptidase. J Biol Chem 248:8366–8369
Hayashi R, Bai Y, Hata T (1975) Evidence for an essential histidine in carboxypeptidase Y. J Biol Chem 250:5221–5226
Horiuchi H, Yanai K, Okazaki T, Takagi M, Yano K (1988) Isolation and sequencing of a genomic clone encoding aspartic proteinase of Rhizopus niveus. J Bacteriol 170:272–278
Ichishima E (1972) Purification and characterization of a new type of acid carboxypeptidase from Aspergillus. Biochim Biophys Acta 258:274–288
Innis MA, Holland MJ, McCabe PC, Cole GE, Wittman VP, Tal R, Watt KWK, Gelfand DH, Holland JP, Meade JH (1985) Expression, glycosylation, and secretion of an Aspergillus glucoamylase by Saccharomyces cerevisiae. Science 228:21–26
Langford CJ, Klinz FJ, Donath C, Gellwitz D (1984) Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell 36:645–653
Lee BR, Takeuchi M, Kobayashi Y (1993) Purification and characterization of serine carboxypeptidases from Absidia zychae. Biosci Biotech Biochem 57:618–622
Liao DI, Remington SJ (1990) Structure of wheat serine carboxypeptidase II at 3.5-Å resolution. J Biol Chem 265:6528–6531
Liao DI, Breddam K, Sweet RM, Bullock T, Remington SJ (1992) Refined atomic model of wheat serine carboxypeptidase II at 2.2-Å resolution. Biochemistry 31:9796–9812
Matsudaira P (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262:10035–10038
Nunberg JH, Meade JH, Cole G, Lawyer FC, McCabe P, Schweickart V, Tal R, Wittman VP, Flatgaard JE, Innis MA (1984) Molecular cloning and characterization of the glucoamylase gene of Aspergillus awamori. Mol Cell Biol 4:2306–2315
Olesen K, Kielland-Brandt MC (1993) Altering substrate preference of carboxypeptidase Y by a novel strategy of mutagenesis eliminating wild-type background. Protein Eng 6:409–415
Peberdy JF (1994) Protein secretion in filamentous fungi; trying to understand a highly productive black box. Trends Biotechnol 12:50–57
Sakaguchi K, Takagi M, Horiuchi H, Gomi K (1992) Fungal enzymes used in oriental food and beverage industries In: Kinghorn JR, Turner G (eds) Applied molecular genetics of filamentous fungi. Blackie Academic and Professional, Glasgow, UK, pp 54–99
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning (2nd edn.) Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schechman MG, Yanofsky C (1983) Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet 2:83–99
Shoemaker S, Schweickart V, Ladner M, Gelfand D, Kwok S, Myambo K, Innis M (1983) Molecular cloning of exo-cellobiohydrolase I derived from Trichoderma reesei strain L27. Bio/Technol 1:691–696
Sørensen SB, Breddam K, Svendsen I (1986) Primary structure of carboxypeptidase I from malted barley. Carlsberg Res Commun 51:475–485
Sørensen SB, Svendsen I, Breddam K (1987) Primary structure of carboxypeptidase II from malted barley. Carlsberg Res Commun 52:285–295
Sørensen SB, Svendsen I, Breddam K (1989) Primary structure of carboxypeptidase III from malted barley. Carlsberg Res Commun 54:193–202
Takeuchi M, Ichishima E (1981) Mode of action of a new Aspergillus oryzae carboxypeptidase O-1. Agric Biol Chem 45: 1033–1035
Takeuchi M, Ichishima E (1986) A 155 k acid carboxypeptidase O from Aspergillus oryzae. Agric Biol Chem 50:633–638
Takeuchi M, Ichishima E (1989) Action of serine carboxypeptidase from Aspergillus saitoi on carboxyterminal amidated peptides. Agric Biol Chem 53:2301–2306
Tan F, Morris PW, Skidgel RA, Erdos EG (1993) Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). J Biol Chem 268:16631–16638
Valls LA, Hunter CP, Rothman JH, Stevens TH (1987) Protein sorting in yeast: The localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell 48:887–897
Washio K, Ishikawa K (1992) Structure and expression during the germination of rice seeds of the gene for a carboxypeptidase. Plant Mol Biol 19:631–640
Widmer F, Breddam K, Johansen JT (1981) Influence of the structure of amine components on carboxypeptidase Y-catalyzed amide bond formation. Carlsberg Res Commun 46:97–106
Wöstemeyer J, Burmester A, Weigel C (1987) Neomycin resistance as a dominantly selectable marker for transformation on the zygomycete Absidia glauca. Curr Genet 12:625–627
Yokoyama S, Oobayashi A, Tanabe O, Ichishima E (1974) Submerged production, purification, and crystallization of acid carboxypeptidase from Penicillium janthinellum. Appl Microbiol 28:742–747
Young RA, Davis RW (1983) Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci USA 80:1194–1198
Author information
Authors and Affiliations
Additional information
Communicated by M. Yamamoto
Rights and permissions
About this article
Cite this article
Lee, B.R., Takeuchi, M. & Kobayashi, Y. Molecular cloning and sequence analysis of the scpZ gene encoding the serine carboxypeptidase of Absidia zychae . Curr Genet 27, 159–165 (1995). https://doi.org/10.1007/BF00313430
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00313430