Abstract
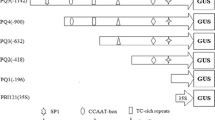
A genomic clone encoding the γ-kafirin gene from sorghum was isolated and sequenced. A 2938 bp sequenced fragment includes an intronless open reading frame of 636 nucleotides encoding a putative polypeptide of 212 amino acids. Comparison of the deduced amino acid sequence of γ-kafirin with the published sequences of γ-prolamins of maize, and Coix revealed highly conserved domains. The N-terminal region of these proteins contains the conserved hexapeptide PPPVHL, which is repeated eight times in γ-zein, four times in γ-kafirin and three times in γ-coixin. The number of PPPVHL repeats accounts predominantly for the differences in the molecular weights of γ-prolamins. Several putative regulatory sequences common to the γ-kafirin and γ-zein genes were identified in both the 5′ and the 3′ flanking regions. Putative GCN4-like regulatory sequences were found at positions −192 and −476 in the 5′ flanking region of γ-kafirin. In the 3′ noncoding region, three putative polyadenylation signals, two AATAAT and one AATGAA, were found at positions + 658, + 716, and + 785, respectively. In order to investigate the role of the putative GCN4-like motifs and other possible cis-acting element(s) of the γ-kafirin promoter, a series of deleted and chimeric promoter constructs were introduced into maize, Coix and sorghum tissues by particle bombardment. Histochemical analysis of β-glucuronidase (GUS) activity in different tissues indicated that the element(s) responsible for tissue specificity is probably located in the 285-bp proximal region of the promoter, while the remaining promoter sequence seems to carry the element(s) responsible for the quantitative response.
Similar content being viewed by others
References
Barros EG, Takasaki K, Kirleis AW, Larkins BA (1991) Nucleotide sequence of a cDNA clone encoding γ-kafirin protein from Sorghum bicolor. Plant Physiol 97:1606–1607
Benfey PN, Chua N-H (1990) The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250:959–966
Benton WD, Davis RW (1977) Screening λgt recombinant clones by hybridization to single plaques in situ. Science 196:180–182
Brown JWS, Wandelt C, Feix G, Neuhaus G, Schweiger HG (1986) The upstream regions of zein genes - Sequence analysis and expression in the unicellular alga Acetabularia. Eur J Cell Biol 42:161–170
Colot V, Robert LS, Kavanagh TA, Bevan MW, Thompson RD (1987) Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J 6:3559–3564
Das PD, Messing JW (1987) Allelic variation and differential expression at the 27-kilodalton zein locus in maize. Mol Cell Biol 7:4490–4497
Das PO, Ward K, Ray S, Messing J (1991a) Sequence variation between alleles reveals two types of copy correction at the 27-kDa zein locus of maize. Genomics 11:849–856
Das PO, Poliak E, Ward K, Messing J (1991b) A new allele of the duplicated 27kD zein locus of maize generated by homologous recombination. Nucleic Acids Res 19:3325–3330
DeRose RT, Ma D-P, Kwon I-S, Hasnain SE, Klassy RC, Hall TC (1989) Characterization of the kafirin gene family from sorghum reveals extensive homology with zein from maize. Plant Mel Biol 12:245–256
Esen A (1986) Separation of alcohol-soluble proteins (zeins) form maize into three fractions by differential solubility.Plant Physiol 80:623–627
Esen A, Bietz JA, Paulis JW, Wall JS (1982) Tandem repeats in the N-terminal sequence of a proline-rich protein from corn endosperm. Nature 296:678–679
Evans DJ, Schiissler L, Taylor JRN (1987) Isolation of reducedsoluble protein from sorghum starchy endosperm. J Cereal Sci 5:61–65
Fang R-X, Nagy F, Sivasubramaniam S, Chua N-H (1989) Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell 1:141–150
Gallardo D, Reina M, Rigua J, Boronat A, Palau J (1988) Genomic organization of the 28 kDa glutelin-2 gene from maize. Plant Sci 54:211–218
Gallie DR, Sleat DE, Wats JW, Turner PC, Wilson TMA (1987) A comparison of eukaryiotic viral 5′-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res 15:8693–8711
Hammond-Kosack MCU, Holdsworth MJ, Bevan MW (1993) In vivo footprinting of a low molecular weight glutenin gene (LMWF-1D1) in wheat endosperm. EMBO J 12:545–554
Higgins DG, Sharp PM (1989) Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS 5:151–153
Hill DE, Hope IA, Macke JP, Struhl K (1986) Saturation mutagenesis of the yeast his3 regulatory site: Requirements for transcriptional induction and for binding by GCN4 activator protein. Science 234:451–457
Hull GA, Halford NG, Kreis M, Shewry PR (1991) Isolation and characterization of genes encoding rye prolamins containing a highly repetitive sequence motif. Plant Mol Biol 17:1111–1115
Jefferson RA (1987) Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol 5:387–405
Johnson DA, Gautsch JW, Sportsman JR, Elder JH (1984) Improved technique utilizing non-fat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal Techniques 1:3–8
Joshi CP (1987) Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res 15:9627–9640
Kirihara JA, Husperger JP, Mahoney JW (1988) Differential expression of a gene for a methionine-rich storage protein in maize. Mol Gen Genet 211:477–484
Kreis M, Williamson MS, Forde J, Schmutz D, Clark J, Buxton B, Pywell J, Marris C, Henderson J, Harris N, Shewry PR, Forde BG, Miflin BJ (1986) Differential gene expression in the developing barley endosperm. Philos Trans R Soc Lond [Biol] 314:355–356
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–684
Leite A, Ottoboni LMM, Targon MLPN, Silva MJ, Arruda P (1990) Phylogenetic relationships of zeins and coixins as determined by immunological cross-reactivity and Southern Blot analysis. Plant Mol Biol 14:743–751
Leite A, Freitas FA, Yunes JA, Arruda P (1991) Nucleotide sequence of a cDNA clone encoding γ-coixin from Coix lacryma jobi seeds. Plant Physiol 97:1604–1605
Leite A, Yunes JA, Turcinelli SR, Arruda P (1992) Cloning and characterization of a cDNA encoding a sulfur-rich coixin. Plant Mol Biol 18:171–174
Liu C-N, Rubenstein I (1992) Molecular characterization of two types of 22 kilodalton α-zein genes in a gene cluster in maize. Mol Gen Genet 234:244–253
Marks MD, Lindell JS, Larkins BA (1985) Nucleotide sequence analysis of mRNAs from maize endosperm. J Biol Chem 260:16451–16459
Moreno MR, Smith JF, Smith RV (1985) Silver staining of proteins in polyacrylamide gels: Increased sensibility through a combined Coomassie Blue-silver stain procedure. Anal Biochem 151:466–470
Müller M, Knudsen S (1993) The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and prolamin box. Plant J 4:343–355
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Ottoboni LMM, Leite A, Yunes JA, Targon MLPN, Souza Filho GA, Arruda P (1993) Sequence analysis of 22 kDA-like α-coixin genes and their comparison with homologous zein and kafirin genes reveals highly conserved protein structure and regulatory elements. Plant Mol Biol 21:765–778
Pedersen K, Argos P, Naravana SLV, Larkins BA (1986) Sequence analysis and characterization of a maize gene encoding a highsulfur zein protein of M r 15000. J Biol Chem 14:6279–6284
Plikaytis BD, Carlone GM, Edwards P, Mayer W (1986) Robust estimation of standard curves for protein molecular weight and linear-duplex DNA based pair number after gel electrophoresis. Anal Biochem 152:346–364
Prat S, Cortadas J, Puigdomenech P, Palau J (1985) Nucleic Acid (cDNA) and amino acid sequences of the maize endosperm protein glutelin-2. Nucleic Acids Res 13:1493–1504
Prat S, Pérez-Grau L, Puigdomènech P (1987) Multiple variability in the sequence of a family of maize endosperm proteins. Gene 52:41–49
Proudfoot N (1991) Poly(A) signals. Cell 64:671–674
Quayle T, Feix G (1992) Functional analysis of the — 300 region of maize zein genes. Mol Gen Genet 231:369–374
Quayle TJA, Hetz W, Feix G (1991) Characterization of a maize endosperm culture expressing zein genes and its use in transient transformation assays. Plant Cell Rep 9:544–548
Rackwitz HR, Zehetner G, Fischauf AM, Lehrach H (1984) Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene 30:195–200
Reina M, Guillén P, Ponte I, Boronat A, Palau J (1990a) DNA sequence of the gene encoding the Zc1 protein from Zea mays W64 A. Nucleic Acids Res 18:6425
Reina M, Ponte I, Guillén P, Boronat A, Palau J (1990b) Sequence analysis of a genomic clone encoding a Zc2 protein from Zea mays W64 A. nucleic Acids Res 18:6426
Rivin CJ, Zinner EA, Walbot V (1982) Isolation of DNA and -1DNA recombinants from maize. In: Sheridan WF (ed) Maize for biological research. University Press, University of North Dakota, Grand Forks, pp 161–164
Shewry PR, Tatham AS (1990) The prolamin storage of cereal seeds: structure and evolution. Biochem J 267:1–12
Steel RGD, Torrie JH (1980) Principles and procedures of statistics. A biometrical approach. McGraw-Hill, New York, pp 172–194
Summer-Smith M, Rafalski JA, Sugyama T, Stoll M, Söll D (1985) Conservation and variability of wheat α/β-gliadin genes. Nucleic Acids Res 13:3905–3916
Taylor JRN, Von Benecke R, Carlsson FHH (1989) Distribution, purification and N-terminal amino acid sequence of sorghum reduced-soluble protein. J Cereal Sci 9:169–177
Thompson GA, Boston RS, Lzynik LA, Hodges TK, Larkins BA (1990) Analysis of promoter activity from an α-zein gene 5′ flanking sequence in transient expression assays. Plant Mol Biol 15:755–764
Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss H-H (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15:5890
Wang S-Z, Esen A (1986) Primary structure of a prolamin-rich zein and its cDNA. Plant Physiol 81:70–74
Wu L, Ueda T, Messing (1993) 3′-end processing of the maize 27 kDa zein mRNA. Plant J 4:535–544
Ye G-N, Daniell H, Sanford JC (1990) Optimization of delivery of foreign DNA into higher plant chloroplasts. Plant Mot Biol 15:809–819
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
The nucleotide sequence data reported in this paper appear in the EMBL/GenBank/DDBJ Nucleotide Sequence Databases under the accession number X62480
Rights and permissions
About this article
Cite this article
de Freitas, F.A., Yunes, J.A., da Silva, M.J. et al. Structural characterization and promoter activity analysis of the γ-kafirin gene from sorghum. Molec. Gen. Genet. 245, 177–186 (1994). https://doi.org/10.1007/BF00283265
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283265