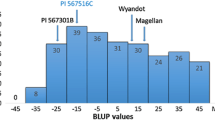
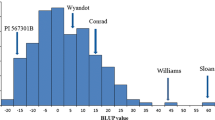
Abstract
A major partial-resistance locus to the soybean cyst nematode (Heterodera glycines Ichinohe; SCN) was identified on linkage group ‘G’ of soybean [Glycine max (L.) Merr.] using restriction fragment length polymorphisms (RFLPs). This locus explained 51.4% (LOD=10.35) of the total phenotypic variation in disease response in soybean Plant Introduction (PI) 209332, 52.7% (LOD=15.58) in PI 90763, 40.0% (LOD=10.50) in PI 88788, and 28.1% (LOD=6.94) in ‘Peking’. Initially, the region around this major resistance locus was poorly populated with DNA markers. To increase marker density in this genomic region, first random, and later targeted, comparative mapping with RFLPs from mungbean [Vigna radiata (L.) R. Wilcz.] and common bean (Phaseolus vulgaris L.) was performed, eventually leading to one RFLP marker every 2.6 centimorgans (cM). Even with this marker density, the inability to resolve SCN disease response into discrete Mendelian categories posed a major limitation to mapping. Thus, qualitative scoring of SCN disease response was carried out in an F5∶6 recombinant inbred population derived from ‘Evans’xPI 209332 using a 30% disease index cut-off for resistance. Using the computer program JoinMap, an integrated map of the region of interest was created, placing the SCN resistance locus 4.6 cM from RFLP marker B53 and 2.8 cM from Bng30. This study demonstrates how a combination of molecularmapping strategies, including comparative genome analysis, join mapping, and qualitative scoring of a quantitative trait, potentially provide the necessary tools for high-resolution mapping around a quantitative-trait locus.
Similar content being viewed by others
References
Arumuganathan K, Earle E (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Anand SC, Brar GS (1983) Response of soybean lines to differently selected cultures of soybean cyst nematode Heterodera glycines Ichinohe. J Nematol 15:120–123
Anand SC, Gallo KM (1984) Identification of additional soybean germ plasm with resistance to race 3 of the soybean cyst nematode. Plant Dis 68:593–595
Anand SC, Wrather JA, Shumway CR (1985) Soybean genotypes with resistance to races of the soybean cyst nematode. Crop Sci 25:1073–1075
Bonierbale MW, Plaisted RL, Tanskley SD (1988) RFLP maps based on common sets of clones reveal modes of chromosome evolution in potato and tomato. Genetics 120:1095–1103
Boutin S, Ansari H, Concibido V, Denny R, Orf J, Young N (1992) RFLP analysis of cyst nematode resistance in soybeans. Soybean Genet Newslett 19:123–127
Boutin S, Young N, Yu ZH, Shoemaker R, Vallejos CE (1995) Genome conservation among three legume genera detected with DNA markers. Genome 38:928–937
Chase CD, Ortega VM, Vallejos CE (1990) DNA restriction fragment length polymorphisms correlate with isozyme diversity in Phaseolus vulgaris L. Theor Appl Genet 81:806–811
Concibido VC (1995) Identification and characterization of SCN resistance genes using DNA markers. PhD thesis, University of Minnesota
Concibido VC, Denny RL, Boutin SR, Hautea R, Orf JH, Young ND (1994) DNA marker analysis of loci underlying resistance to soybean cyst nematode (Heterodera glycines Ichinohe). Crop Sci 34:240–246
Concibido VC, Denny R, Lange D, Danesh D, Orf J, Young N (1995) The soybean cyst nematode resistance gene on linkage group ‘G’ is common among sources of resistance. Soybean Genet Newslett 22:269–272
Danesh D, Aarons S, McGill GE, Young ND (1994) Genetic dissection of oligogenic resistance to bacterial wilt in tomato. Mol Plant-Microbe Interact 7:464–471
Danesh D, Young N, Concibido V, Orf J (1995) Physical mapping of DNA markers near a major soybean cyst nematode resistance locus. Soybean Genet Newslett 22:273–277
Dellaporta SL, Wood I, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 5:19–21
DeScenzo RA, Wise RP, Mahadevappa M (1994) High-resolution mapping of the Hor1/Mla/Hor2 region on chromosome 5S in barley. Mol Plant-Microbe Interact 7:657–666
Doebley J, Stec A (1991) Genetic analysis of the morphological differences between maize and teosinte. Genetics 129: 285–295
Fatokun CA, Menancio-Hautea DI, Danesh D, Young ND (1992) Evidence for orthologous seed-weight genes in cowpea and mungbean based on RFLP mapping. Genetics 132:841–846
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem 132:9
Ferreira AR, Clayton K, Webb DM, Keim P (1994) Multiple locus RFLP-designed bulk segregant analysis of a soybean cyst nematode resistance QTL. In: Heller SR, Gale MD, Miksche JP, Takayanagi K (eds) Plant genome III. Scherago International, San Diego, Calif., p 28
Geiger HH, Heun M (1989) Genetics of quantitative resistance to fungal diseases. Annu Rev Phytopathol 27:317–341
Golden AM, Epps JM, Riggs RD, Duclos LA, Fox JA, Bernard RL (1970) Terminology and identity of infraspecific forms of the soybean cyst nematode (Heterodera glycines). Plant Dis Rep 54:544–546
Hartwig EE (1985) Breeding productive soybeans with resistance to soybean cyst nematode. In: Shibles RA (ed). World Soybean Res.Conf. III. Westview Press, Boulder, Colo., pp. 394–399
Hauge BM, Hanley SM, Cartinhour S, Cherry JM, Goodman HM, Koorneef M, Stam P, Chang C, Kempin S, Medrano L, Meyerwitz EM (1993) An integrated genetic/RFLP map of the Arabidopsis thaliana genome. Plant Jour 3:745–754
Helentjaris T (1993) Implications for conserved genomic structure among plant species. Proc Natl Acad Sci USA 90:353–363
Islam MR, Shepherd KW (1991) Analyses of phenotypes of recombinants and revertants from test cross progenies involving genes at the L group, conferring resistance to rust in flax. Heriditas 114:125–129
Keim P, Shoemaker RC, Palmer RG (1989) Restriction fragment length polymorphism diversity in soybean. Theor Appl Genet 77:786–792
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12: 172–175
Lander ES, Botstein D (1989) Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE (1987) Mapmaker: an interactive computer package for constructing genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spirey R, Wu T, Earle ED, Tanksley SD (1993) Map-based cloning of a protein kinase gene conferring resistance in tomato. Science 262: 1432–1435
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA. 88:9828–9832
Menancio-Hautea D, Kumar L, Danesh D, Young ND (1993) A genome map for mungbean [Vigna radiata (L.) Wilczek] based on DNA genetic markers (2N=2X=22). In: O'Brien SJ (ed) Genetic maps: locus maps of complex genomes. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 6.259–6.261
Nodari RO, Tsai SM, Gilbertson RL, Gepts P (1993) Toward an integrated linkage map of common bean. 2. Development of an RFLP-based linkage map. Theor Appl Genet 85:513–520
Paran I, Kesseli R, Michelmore R (1991) Identification of restriction fragment length polymorphism and random amplified polymorphic DNA markers linked to downey mildew resistance genes in lettuce, using near-isogenic lines. Genome 34:1021–1027
Paterson AH, Lander ES, Hewitt JD, Paterson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorpisms. Nature 335:721–726
Paterson AH, DeVerna JW, Lanini B, Tanksley SD (1990) Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes, in an interspecies cross of tomato. Genetics 124:735–742
Paterson A, Damon S, Hewitt JD, Zamir D, Rabinowitch HD, Lincoln SE, Lander ES, Tanksley SD (1991) Mendelian factors underlying quantitative traits in tomato: comparison across species, generations, and environments. Genetics 127:181–197
Pereira MG, Lee M, Bramel-Cox P, Woodman W, Doebley J, Whitkus R (1994) Construction of an RFLP map in sorghum and comparative mapping in maize. Genome 37:236–243
Phillips RL, Kim TS, Kaeppler SM, Parentoni SN, Shaver DL, Stucker RE, Openshaw SJ (1993) Genetic dissection of maturity using RFLPs. In: Proc 47th Annu Corn Sorghum Ind Res Conf. American Seed Trade Association, Chicago Ill. pp 135–150
Saiki RK, Gelfand DH, Stoffel S, Scharf S, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Schmitt DP, Shannon G (1992) Differentiating soybean to Heterodera glycines races. Crop Sci 32:275–277
Shoemaker RC, Olson TC (1993) Molecular linkage map of soybean (Glycine max L. Merr.) In: O'Brien SJ (ed) Genetic maps: locus maps of complex genomes. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 6.131–6.138
Shoemaker RC, Specht JE (1995) Integration of the soybean molecular and classical genetic linkage groups. Crop Sci 35:436–446
Song KM, Osborn TC, Williams PH (1990) Brassica taxonomy based on nuclear RFLPs. 3. Genome relationships in Brassica and related genera and the orgin of B. oleracea and B. rapa (syn. campestris). Theor Appl Genet 79:9
Southern E (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant Jour 3:739–744
Sudupak MA,Bennetzen JL, Hulbert SH (1993) Unequal exchange and meiotic instability of disease resistance genes in the Rp1 region of maize. Genetics 132:119–125
Tanksley SD (1993) Mapping polygenes. Annu Rev Genet 27:205–233
Tanksley SD, Bernatzky R, Lapitan NL, Prince JP (1988) Conservation of gene repertoire but not gene order in pepper and tomato. Proc Natl Acad Sci USA 85:6419–6423
Tanksley SD, Ganal MW, Martin GB (1995) Chromosome landing: a paradigm for map-based gene cloning in plants with large genomes. Trends Genet 11 63–68
Vallejos CE, Sakiyama NS, Chase CD (1992) A molecular markerbased linkage map of Phaseolus vulgaris L. Genetics 131:733–740
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Webb DE, Baltazar BM, Rao Arelli AO, Schupp J, Clayton K, Keim P, Beavis WD (1995) Genetic mapping of soybean cyst-nematode race-3 resistance loci in the soybean PI-437.654. Theor Appl Genet 91:74–581
Weeden NF, Muehlbauer FJ, Ladizinsky G (1992) Extensive conservation of linkage relationships between pea and lentil genetic maps. J Hered 83:123–29
Williams J, Kubelik A, Livak K, Rafalski J, Tingey S (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Young ND, Kumar L, Menancio-Hautea D, Danesh D, Talekar NS, Shanmugasundarum S, Kim DH (1992) RFLP mapping of a major bruchid resistance gene in mungbean (Vigna radiata L. Wilczek). Theor Appl Genet 84:839–844
Author information
Authors and Affiliations
Additional information
Communicated by G. Wenzel
Rights and permissions
About this article
Cite this article
Concibido, V.C., Young, N.D., Lange, D.A. et al. Targeted comparative genome analysis and qualitative mapping of a major partial-resistance gene to the soybean cyst nematode. Theoret. Appl. Genetics 93, 234–241 (1996). https://doi.org/10.1007/BF00225751
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00225751