Abstract
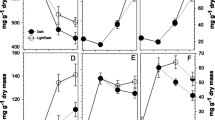
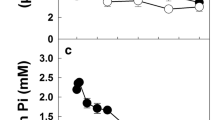
(i) Sucrose-phosphate synthase (SPS) was purified 40-fold from stored potato (Solanum tuberosum L.) tubers to a final specific activity of 33–70 nkat·(mg protein)−1 via batch elution from diethylaminoethyl (DEAE)-sephacel, polyethylene glycol (PEG) precipitation and Mono Q anion-exchange chromatography. (ii) Immunoblotting revealed a major and a minor band with molecular weights of 124.8 kDa and 133.5 kDa, respectively. Both bands were also present in extracts prepared in boiling SDS to exclude proteolysis. No smaller polypeptides were seen, except when the preparations were incubated before application on a polyacrylamide gel. (iii) The enzyme preparation was activated by glucose-6-phosphate and inhibited by inorganic phosphate. Both effectors had a large effect on the K m (fructose-6-phosphate) and the K m (uridine-5-diphosphoglucose) with phosphate acting antagonistically to glucose-6-phosphate. (iv) Preincubation of potato slices with low concentrations of okadaic acid or microcystin resulted in a three- to fourfold decrease in the activity of SPS when the tissue was subsequently extracted and assayed. The decrease was especially marked when the assay contained low concentrations of substrates and glucose-6-phosphate, and inorganic phosphate was included. Preincubation with mannose or in high osmoticum resulted in an increase of SPS activity. (v) Analogous changes were observed in germinating Ricinus communis L. seedlings. After preincubation of the cotyledons in glucose, high SPS activity could be measured, whereas okadaic acid, omission of glucose, or addition of phosphate or sucrose led to a large decrease of SPS activity in the “selective” assay. (vi) It is argued that SPS from non-photosynthetic tissues is regulated by metabolites and by protein phosphorylation in an analogous manner to the leaf enzyme.
Similar content being viewed by others
Abbreviations
- Fru6P:
-
fructose-6-phosphate
- Glc6P:
-
glucose-6-phosphate
- Pi:
-
inorganic phosphate
- PGI:
-
phosphoglucose isomerase
- PP2A:
-
phosphoprotein phosphatase 2A
- PEG:
-
polyethyleneglycol
- SPS:
-
sucrose-phosphate synthase
- UDPGlc:
-
uridine-5-diphosphoglucose
References
Bruneau, J.-M., Worrell, A.C., Cambou, B., Lando, D., Voelker, T.A. (1991) Sucrose phosphate synthase, a key enzyme for sucrose biosynthesis in plants. Plant Physiol. 96, 473–478
Cardini, C.E., Slabnik, E., Frydman, R.B. (1968) Some properties of potato tuber UDP glucose: d-fructose-2-glucosyltransferase and UDP glucose: d-fructose-6-phosphate-2-glucosyltransferase. Plant Physiol. 43, 1063–1068
Dancer, J.E., Matzfeld, W.D., Stitt, M. (1990) Cytosolic cycles regulate the turnover of sucrose in heterotrophic cell-suspension cultures of Chenopodium rubrum L. Planta 182, 223–251
Doehlert, D.C., Huber, S.C. (1983) Regulation of spinach leaf sucrose-phosphate synthase by glucose-6-phosphate, inorganic phosphate and pH. Plant Physiol. 73, 989–994
Doehlert, D.C., Huber, S.C. (1985) The role of sulfhydryl groups in the regulation of spinach leaf sucrose-phosphate synthase. Biochim. Biophys. Acta 830, 276–273
Geigenberger, P., Stitt, M. (1991) A futile cycle of sucrose synthase and degradation is involved in regulating partitioning between sucrose, starch and respiration in cotyledons of germinating Ricinus communis L. seedlings when phloem transport is inhibited. Planta 185, 81–90
Geigenberger, P., Stitt, M. (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189, 329–339
Guy, C.L. (1990) Cold acclimation and freezing stress tolerance. Annu. Rev. Plant Physiol. Mol. Biol. 41, 187–223
Hajirezeai, M., Stitt, M. (1991) Contrasting roles for pyrophosphate: fructose-6-phosphate phosphotransferase during aging of tissue slices from potato tubers and carrot slices. Plant Sci. 77, 177–183
Hawker, J.S. (1985) Sucrose. In: The biochemistry of storage carbohydrates in green plants, pp. 1–51, Dey, P.M., Dixon, R.A. eds. Academic Press, New York
Hubbard, N.L., Pharr, M.D., Huber S.C. (1990) Role of sucrose phosphate synthase in ripening bananas and its relation to the respiratory climacteric. Plant Physiol. 94, 201–208
Huber, S.C., Huber, J.L.A. (1991) Regulation of maize leaf sucrose-phosphate synthase by protein phosphorylation. Plant Cell Physiol. 32, 319–326
Huber, S.C., Huber, J.L.A., Nielsen, T.H. (1989) Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch. Biochem. Biophys. 270, 681–690
Jelitto, T., Sonnewald, U., Willmitzer, L., Hajirezeai, M., Stitt, M. (1992) Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188, 238–244
Kalt-Torres, W., Kerr, P.S., Huber, S.C. (1987) Isolation and characterization of multiple forms of maize leaf sucrose-phosphate synthase. Plant Physiol. 70, 653–658
Krüger, N. (1990) Carbohydrate synthesis and degradation. In: Plant physiology, biochemistry and molecular biology, pp. 59–76, Dennis, D.T., Turpin, D.M., eds. Lorgmar, Singapore
MacRae, E., Quick, W.P., Benker, C., Stitt, M. (1992) Carbohydrate metabolism during ripening of kiwi fruit. Planta 188, 314–323
Neuhaus, H.E., Quick, W.P., Siegl, G., Stitt, M. (1990) Control of photosynthate partitioning in spinach leaves. Planta 181, 583–592
Nomura, T., Akazawa, T. (1974) Enzyme mechanism of starch breakdown in germinating rice seeds. V. Sucrose phosphate synthase in the scutellum. Plant Cell Physiol. 15, 477–483
Plaxton, W.C., Preiss, J. (1987) Purification and properties of non-proteolytic degraded ADP glucose pyrophosphorylase from maize endosperm. Plant Physiol. 83, 105–112
Quick, W.P., Siegl, G., Neuhaus, H.E., Stitt, M. (1989) Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose phosphate synthase. Planta 177, 535–546
Salerno, G.L., Pontis, H.G. (1978) Sucrose phosphate synthase, separation from sucrose synthase and study of its properties. Planta 142, 41–48
Salvucci, M.E., Drake, R.R., Haley, B.E. (1990) Purification and photoaffinity labelling of sucrose-phosphate synthase from spinach leaves. Arch. Biochem. Biophys. 281, 212–218
Siegl, G., Mackintosh, C., Stitt, M. (1990) Sucrose phosphate synthase is dephosphorylated by protein phosphatase 2A in spinach leaves. FEBS Lett. 270, 198–202
Siegl, G., Stitt, M. (1990) Partial purification of two forms of spinach leaf sucrose-phosphate synthase which differ in their kinetic properties. Plant Sci. 66, 205–210
Sonnewald, U., Quick, W.P., MacRae, E., Krause, K.-P., Stitt, M. (1993) Purification, cloning and expression of spinach leaf sucrose-phosphate synthase in E. coli. Planta 189, 174–181
Stitt, M., Huber, S.C., Kerr, P. (1987) Control of photosynthetic sucrose synthesis. In: The biochemistry of plants, vol.10, pp. 328–429, Hatch, M.D., Boardman, N.R. eds. Academic Press, New York
Stitt, M., Wilke, I., Feil, R., Heldt, H.W. (1988) Coarse control of sucrose phosphate synthase in leaves: alterations of the kinetic properties in response to the rate of photosynthesis and accumulation of sucrose. Planta 174, 217–230
Walker, J.L.A., Huber, S.C. (1989a) Purification and preliminary characterization of sucrose-phosphate synthase using monoclonal antibodies. Plant Physiol. 89, 518–524
Walker, J.L.A., Huber, S.C. (1989b) Regulation of sucrose-phosphate synthase activity in spinach leaves by protein level and covalent modification. Planta 117, 116–120
Weiner, H., McMichael, R.W., Huber, S.C. (1992) Identification of factors regulating the phosphorylation status of sucrose-phosphate synthase in vivo. Plant Physiol. 99, 1435–1442
Zrenner, R., Stitt, M. (1991) Comparison of the effect of rapidly and gradually developing water-stress on carbohydrate metabolism in spinach leaves. Plant Cell Environ. 14, 939–946
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft, the BMFT and Sandoz AG, Basel, Switzerland. We are grateful to Prof. E. Beck (Pflanzenphysiologie, Bayreuth, Germany) for providing us with laboratory facilities, and to Dr. U. Sonnewald (Institut für Genbiologische Forschung, Berlin, Germany) for many discussions and providing us with unpublished data.
Rights and permissions
About this article
Cite this article
Reimholz, R., Geigenberger, P. & Stitt, M. Sucrose-phosphate synthase is regulated via metabolites and protein phosphorylation in potato tubers, in a manner analogous to the enzyme in leaves. Planta 192, 480–488 (1994). https://doi.org/10.1007/BF00203585
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203585