Summary
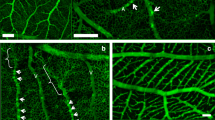
The aim of our investigations was to test whether the chicken chorio-allantoic membrane (CAM) could be an adequate in vivo model for a new mode of capillary growth, originally described in the rat lung and termed intussusceptive microvascular growth. According to that concept the capillary system does not grow by sprouting of vessels, but expands by insertion of transcapillary tissue pillars or posts which form new intercapillary meshes. In the present study, we observed slender transcapillary tissue pillars with diameters around 1 μm in the CAM by in vivo microscopy, and analyzed their ultrastructure by transmission electron microscopic investigation of serial sections. The pillars corresponded in size to those previously described in rat lung microvasculature. On day 7, the pillar core contained endothelial-, endothelial-like cells and collagen fibers, and on day 12 additionally chorionic epithelial cells. As a hypothesis we propose that slender cytoplasmic extensions of endothelial cells, heavily interdigitated in the post area and often projecting into the vascular lumen, could initiate the first step of pillar formation, i.e., interconnect opposite capillary walls. During both stages of development endothelial-like cells were observed in close relationship with the pillars. These cells seem to be relevant for tissue post completion and growth, as they were found to invade the core of the pillars. From the localization of the interendothelial junctions in the post region, a certain similarity to the concept proposed for the lung can be found. The observations confirm that the CAM is a very suitable material for the in vivo investigation of intussusceptive capillary growth.
Similar content being viewed by others
References
Auerbach R, Kubai L, Knighton D, Folkman J (1974) A simple procedure for the long-term cultivation of chicken embryos. Dev Biol 41:391–394
Ausprunk DH, Knighton DR, Folkman J (1974) Differentiation of vascular endothelium in the chick chorioallantois: a structural and autoradiographic study. Dev Biol 38:237–248
Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14:53–65
Burri PH, Tarek MR (1990) A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec 228:35–45
Caduff JH, Fischer LC, Burri PH (1986) Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec 216:154–164
Clark ER, Clark EL (1939) Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat 64:251–301
De Fouw DO, Rizzo VJ, Steinfeld R, Feinberg RN (1989) Mapping of the microcirculation in the chick chorioallantoic membrane during normal angiogenesis. Microvasc Res 38:136–147
Dunn BE, Fitzharris TP (1979) Differentiation of the chorionic epithelium of chick embryos maintained in shell-less culture. Dev Biol 71:216–227
Fawcett DW, Wittenberg J (1962) Structural specializations of endothelial cell junctions. Anat Rec 142:231
Fawcett DW (1963) Comparative observations on the fine structure of blood capillaries. In: Orbison L, Smith DE (eds) The peripheral blood vessel. Baltimore, pp 17–44
Frasca J, Parks VR (1965) A routine technique for double staining ultrathin sections using uranyl and lead salts. J Cell Biol 25:157–160
Fujimoto S, Yamamoto K, Takeshige Y (1975) Electron microscopy of endothelial microvilli of large arteries. Anat Rec 183:259–266
Furusato M, Fukunaga M, Kikuchi Y, Chiba S, Yokota K, Job K, Aizawa S, Ishikawa E (1984) Two- and three-dimensional ultrastructural observations of angiogenesis in juvenile hemangioma. Virchows Arch 46:229–237
Furusato M, Wakui S, Suzuki M, Takagi H, Hori M, Asari M, Kano Y, Ushigome S (1990) Three-dimensional ultrastructural distribution of cytoplasmic interdigitation between endothelium and pericyte of capillary in human granulation tissue by serial section reconstruction method. J Electron Microsc 39:86–91
Hoshi H, Mori T (1971) The fine structure of the chorionic epithelium of chick embryo. Arch Histol Jpn 33:45–58
Narbaitz R, Jande SS (1978) Ultrastructural observations on the chorionic epithelium, parathyroid glands and bones from chick embryos developed in shell-less culture. J Embryol Exp Morphol 45:1–12
Needham J (1932) On the true metabolic rate of the chick embryo and the respiration of its membranes. Proc R Soc Lond [Biol] 110:46–74
Nicosia RF, Tchao R, Leighton J (1982) Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro 18:538–549
Patan S, Alvarez MJ, Schittny JC, Burri PH (1992) Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histol Cytol [Suppl] 55:65–75
Pexieder T (1981) Prenatal development of the endocardium: a review. Scanning Electron Microsc II:223–253
Reynolds ES (1963) The use of lead citrate of high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–211
Schoefl GI (1963) Studies on inflammation. III. Growing capillaries: their structure and permeability. Virchows Arch 337:97–141
Sethi N, Brookes M (1971) Ultrastructure of the blood vessels in the chick allantois and chorioallantois. J Anat 109:1–15
Shumko JZ, De Fouw DO, Feinberg RN (1988) Vascular histodifferentiation in the chick chorioallantoic membrane: a morphometric study. Anat Rec 220:179–189
Smith U, Ryan JW, Michie DD, Smith DS (1971) Endothelial projections as revealed by scanning electron microscopy. Science 173:925–927
Sweeny PR, Bather R (1968) An electron microscopic study of the chorioallantoic membrane following infection with rous sarcoma virus. J Cell Biol 36:299–311
Tanaka A (1960) Electron microscopic study on the avian pectines. I. Dobutsugaku Zasshi 69:314–317
Tazawa H (1978) Gas transfer in the chorioallantois. In: Piiper J (ed) Respiration function in birds, adult and embryonic. Springer, Berlin Heidelberg New York, pp 274–291
Thoma R (1893) Untersuchungen über die Histogenese und Histomechanik des Gefässystems. Enke, Stuttgart, pp 1–51
Wagner RC (1980) Endothelial cell embryology and growth. Adv Microcirc, Karger, Basel 9:45–75
Wangensteen D, Weibel ER (1982) Morphometric evaluation of chorioallantoic oxygen transport in the chick embryo. Respir Physiol 47:1–20
Warren BA (1966) The ultrastructure of capillary sprouts induced by melanoma transplants in the golden hamster. J R Microsc Soc 86:177–187
Wilms P, Christ B, Wilting J, Wachtler F (1991) Distribution and migration of angiogenic cells from grafted avascular intraembryonic mesoderm. Anat Embryol 183:371–377
Wolff J (1964) Ein Beitrag zur Ultrastruktur der Blutkapillaren: Das nahtlose Endothel. Z Zellforsch Mikrosk Anat 64:290–300
Yamagami I (1967) Electron microscopic study on the cornea. I. The mechanism of experimental new vessel formation. Acta Soc Ophthalmol Jpn 73:1222–1242
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patan, S., Haenni, B. & Burri, P.H. Evidence for intussusceptive capillary growth in the chicken chorio-allantoic membrane (CAM). Anat Embryol 187, 121–130 (1993). https://doi.org/10.1007/BF00171743
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00171743