Abstract
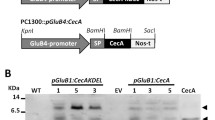
The cDNAs encoding the seed antimicrobial peptides (AMPs) fromMirabilis jalapa (Mj-AMP2) andAmaranthus caudatus (Ac-AMP2) have previously been characterized and it was found that Mj-AMP2 and Ac-AMP2 are processed from a precursor preprotein and preproprotein, respectively [De Bolleet al., Plant Mol Biol 28:713–721 (1995) and 22:1187–1190 (1993), respectively]. In order to study the processing, sorting and biological activity of these antimicrobial peptides in transgenic tobacco, four different gene constructs were made: a Mj-AMP2wild-type gene construct, a Mj-AMP2 mutant gene construct which was extended by a sequence encoding the barley lectin carboxyl-terminal propeptide, a known vacuolar targeting signal [Bednarek and Raikhel, Plant Cell 3: 1195–1206 (1991)]; an Ac-AMP2wild-type gene construct; and finally, an Ac-AMP2 mutant gene construct which was truncated in order to delete the sequence encoding the genuine carboxyl-terminal propeptide. Processing and localization analysis indicated that an isoform of Ac-AMP2 with a cleaved-off carboxyl-terminal arginine was localized in the intercellular fluid fraction of plants expressing eitherwild-type or mutant gene constructs. Mj-AMP2 was recovered extracellularly in plants transformed with Mj-AMP2wild-type gene construct, whereas an Mj-AMP2 isoform with a cleaved-off carboxyl-terminal arginine accumulated intracellularly in plants expressing the mutant precursor protein with the barley lectin propeptide. Thein vitro antifungal activity of the AMPs purified from transgenic tobacco expressing any of the four different precursor proteins was similar to that of the authentic proteins. However, none of the transgenic plants showed enhanced resistance against infection with eitherBotrytis einerea orAlternaria longipes.
Similar content being viewed by others
References
An G, Watson BD, Stachel S, Gordon MP, Nester EW: New cloning vehicles for transformation of higher plants. EMBO J 4: 277–284 (1984).
Bednarek SY, Raikhel NV: The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell 3: 1195–1206 (1991).
Bednarek SY, Raikhel NV: Intracellular trafficking of secretory proteins. Plant Mol Biol 20: 133–150 (1992).
Bednarek SY, Wilkins TA, Dombrowski JE, Raikhel NV: A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell 2: 1145–1155 (1990).
Bevan M: BinaryAgrobacterium vectors for plant transformation. Nucl Acids Res 12: 8711–8721 (1984).
Bohlmann H, Apel K: Thionins. Annu Rev Plant Physiol Plant Mol Biol 42: 227–240 (1991).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GW, Osborn RW: Antimicrobial peptides from plants. Crit Rev Plant Sci, in press (1996)
Broekaert WF, Marien W, Terras FRG, De Bolle MFC, Proost P, Van Damme J, Dillen L, Claeys M, Rees SB, Vanderleyden J, Cammue BPA: Antimicrobial peptides fromAmaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin binding proteins. Biochemistry 31: 4308–4314 (1992).
Broekaert WF, Terras FRG, Cammue BPA, Osborn RW: Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol 108: 1353–1358 (1995).
Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J: An automated quantitative assay for fungal growth inhibition. FEBS Microbiol Lett 69: 55–60 (1990).
Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais J, Broglie R: Transgenic plants with enhanced resistance to the fungal pathogenRhizoctonia solani. Science 254: 1194–1197 (1991).
Cammue BPA, De Bolle MFC, Terras FRG, Proost P, Van Damme P, Rees SB, Vanderleyden J, Broekaert WF: Isolation and characterization of a novel class of plant antimicrobial peptides fromMirabilis jalapa L. seeds. J Biol Chem 267: 2228–2233 (1992).
Cammue BPA, Thevissen K, Hendriks M, Eggermont K, Goderis IJ, Proost P, Van Damme J, Osborn RW, Guerbette F, Kader J-C, Broekaert WF: A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol 109: 445–455 (1995).
Carmona MJ, Molina A, Fernández JA, López-Fando J, Gárcia-Olmedo F: Expression of the α-thionin gene from barley in tobacco confers enhanced resistance to bacterial pathogens. Plant J 3: 457–462 (1993).
Chagolla-Lopez A, Blanco-Labra A, Patthy A, Sanchez R, Ponger S: A novel α-amylase inhibitor from amaranth (Amaranthus hypocondriacus) seeds. J Biol Chem 269: 23675–23680 (1994).
Chrispeels MJ, Raikhel NV: Short peptide domains target proteins to plant vacuoles. Cell 68: 613–616 (1992).
Collinge DB, Slusarenko AJ: Plant gene expression in response to pathogens. Plant Mol Biol 9: 389–410 (1987).
Deblaere R, Reynaerts A, Hofte H, Hernalsteens JP, Leemans J, Van Montagu M: Vector for cloning in plant cells. Meth Enzymol 153: 277–293 (1987).
De Bolle MFC, David KMM, Rees SB, Vanderleyden J, Cammue BPA, Broekaert WF: Cloning and characterization of a cDNA encoding an antimicrobial chitin-binding protein from amaranth,Amaranthus caudatus. Plant Mol Biol 22: 1187–1190 (1993).
De Bolle MFC, Eggermont K, Duncan RE, Osborn RW, Terras FRG, Broekaert WF: Cloning and characterization of two cDNA clones encoding seed-specific antimicrobial peptides fromMirabilis jalapa L. Plant Mol Biol 28: 713–721 (1995).
De Bolle MFC, Terras FRG, Cammue BPA, Rees SB, Broekaert WF:Mirabilis jalapa antimicrobial peptides andRaphanus sativus antifungal proteins: a comparative study of their structure and biological activities. In: Fritig B, Legrand M (eds) Mechanisms of Plant Defense Responses, pp. 433–436. Kluwer Academic Publishers, Dordrecht (1993).
Fernandezde Caleya R, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P: Susceptibility of phytopathogenic bacteria to wheat purothioninsin vitro. Appl Microbiol 23: 998–1000 (1972).
Florack DEA, Dirkse WG, Visser B, Heidekamp F, Stiekema WJ: Expression of biologically active hordothionins in tobacco. Effects of pre- and pro-sequences at the amino and carboxyl termini of the hordothionin precursor on mature protein expression and sorting. Plant Mol Biol 24: 83–96 (1994).
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA: A binary plant vector strategy based on separation ofvir- and T-region ofAgrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180 (1983).
Holwerda BC, Padgett HS, Rogers JC: Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell 4: 307–318 (1992).
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Isaac S: Fungal-plant Interactions. Chapman & Hall, London (1992).
Kay R, Chan A, Daly M, McPherson J: Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299–1302 (1987).
Linthorst HJM: Pathogenesis-related proteins of plants. Crit Rev Plant Sci 10: 123–150 (1991).
Liu D, Raghothama KG, Hasegawa PM, Bressan RA: Osmotin overexpression in potato delays the development of disease symptons. Proc Natl Acad Sci USA 91: 1888–1892 (1994).
Matsuoka K, Nakamura K: Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA 88: 834–838 (1991).
Mauch F, Mauch-Mani B, Boller T: Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1.3-glucanase. Plant Physiol 88: 936–942 (1988).
Melchers LS, Apotheker-de Groot M, van der Knaap JA, Ponstein AS, Sela-Buurlage MB, Bol JF, Cornelissen BJC, Linthorst HJM: A new class of tobacco chitinases homologous to bacterial exo-chitinases displays antifungal activity. Plant J: 469–480 (1994).
Melchers LS, Sela-Buurlage MB, Vloemans SA, Woloshuk CP, van Roekel JSC, Pen J, Van den Elzen PJM, Cornelissen BJC: Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and β-1,3-glucanase in transgenic plants. Plant Mol Biol 21: 586–593 (1993).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Neuhaus J-M, Ahl-Goy P, Hinz U, Flores S, Meins FJr: High-level expression of tobacco chitinase gene inNicotiana sylvestris. Susceptibility of transgenic plants toCercospora nicotianae infection. Plant Mol Biol 16: 141–151 (1991).
Neuhaus J-M, Pietrzak M, Boller T: Mutation analysis of the C-terminal vacuolar targeting peptide of tobacco chitinase: low specificity of the sorting system, and gradual transition between intracellular retention and secretion into the extracellular space. Plant J 5: 45–54 (1994).
Neuhaus J-M, Sticher L, Meins FJr, Boller T: A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA 88: 10362–10366 (1991).
Nielsen KK, Mikkelsen JD, Kragh KM, Bojsen K: An acidic class III chitinase in sugar beet: induction byCercospora beticola, characterization, and expression in transgenic tobacco plants. Mol Plant-Microbe Interact 6: 495–506 (1993).
Odell JT, Nagy F, Chua N-H: Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 (1985).
Rao AG: Antimicrobial peptides. Mol Plant-Microbe Interact 8: 6–13 (1995).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nickler S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, Van den Elzen PJM, Cornelissen BJC: Only specific tobacco (Nicotiana tabacum) chitinases and β-1,3-glucanases exhibit antifungal activity. Plant Physiol 101: 857–863 (1993).
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujitomo EK, Goeke NM, Olson BJ, Klenk DC: Measurement of protein using bicinchonimic acid. Anal Biochem 150: 76–85 (1985).
Sonnewald U, Brauer M, von Schaewen A, Stitt M, Willmitzer L: Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J 1: 95–106 (1991).
Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Broekaert WF: Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7: 573–588 (1995).
Timmermans MCP, Maliga P, Vieira J, Messing J: The pFF plasmids: cassettes utilizing CaMV sequences for expression of foreign genes in plants. J Biotechnol 14: 333–344 (1991).
Van den Elzen PJM, Jongedijk E, Melchers LS, Cornelissen BJC: Virus and fungal resistance: from laboratory to field. Phil Trans R Soc Land B 342: 271–278 (1993).
Vitale A, Chrispeels MJ: Sorting of proteins to the vacuoles of plants. BioEssays 14: 151–160 (1992).
Wada K, Wada Y, Ishibashi F, Gojobori T, Ikemura T: Codon usage tabulated from the GenBank genetic sequence data. Nucl Acids Res 20: 2111–2118 (1992).
Wang H, Qi M, Cutler AJ: A simple method of preparing plant samples for PCR. Nucl Acids Res 17: 4153–4154 (1993).
Wilkins TA, Bednarek SY, Raikhel NV: Role of propeptide glycan in post-translational processing and transport of barley lectin to vacuoles in transgenic tobacco. Plant Cell 2: 301–313 (1990).
Yenofsky RL, Fine M, Pellow JW: A mutant neomycin phosphotransferase II gene reduces the resistance of transformants to antibiotic selection pressure. Proc Natl Acad Sci USA 87: 3435–3439 (1990).
Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ: Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in transgenic tobacco. Bio/technology 12: 807–812 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Bolle, M.F.C., Osborn, R.W., Goderis, I.J. et al. Antimicrobial peptides fromMirabilis jalapa andAmaranthus caudatus: expression, processing, localization and biological activity in transgenic tobacco. Plant Mol Biol 31, 993–1008 (1996). https://doi.org/10.1007/BF00040718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040718