Abstract
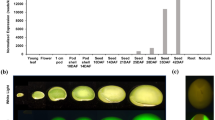
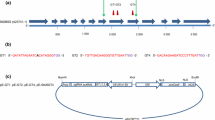
In vitro mutagenesis was used to supplement the sulfur amino acid codon content of a gene encoding β-phaseolin, a Phaseolus vulgaris storage protein. The number of methionine codons in the phaseolin gene was increased from three to nine by insertion of a 45 base pair (bp) synthetic duplex. Either modified or normal phaseolin genes were integrated into the genome of tobacco plants through Agrobacterium tumefaciens-mediated transformation. Although similar levels of phaseolin RNA are detected in seeds of plants transformed with either the normal or modified (himet) gene, the quantity of himet protein is consistently much lower than normal β-phaseolin. Himet phaseolin is expressed in a temporal- and organ-specific fashion, and is N-glycosylated and assembled into trimers in the manner of normal phaseolin. After germination, both types of phaseolin are hydrolyzed, but the himet protein is more quickly degraded. Electron microscopic immunocytochemical observations of developing seeds indicate that the himet protein is primarily localized in the endoplasmic reticulum (ER) and in Golgi apparatus secretion vesicles. Himet phaseolin is absent from protein storage vacuoles, termed protein bodies, where normal phaseolin is deposited in transgenic tobacco. We interpret the immunocytochemical data to indicate that himet phasolin is transported through the ER and Golgi apparatus and is then degraded in Golgi secretion vesicles or the protein bodies.
Similar content being viewed by others
References
Aoki S, Takebe I: Infection of tobacco mesophyll protoplasts by tobacco mosaic ribonucleic acid. Virology 39: 439–448 (1969).
Baumgartner B, Tokuyasu KT, Chrispeels MJ: Immunocytochemical localization of reserve protein in the endoplasmic reticulum of developing bean (Phaseolus vulgaris) cotyledons. Planta 150: 419–425 (1980).
Blagrove RJ, Lilley GG, VanDonkelaar A, Sun SM, Hall TC: Structural studies of a French bean storage protein: phaseolin. Int J Biol Macromol 6: 137–141 (1984).
Bollini R, Chrispeels MJ: Characterization and subcellular localization of vicilin and phytohemagglutinin, the two major reserve proteins of Phaseolus vulgaris L. Planta 142: 291–298 (1978).
Bollini R, Chrispeels MJ: The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146: 487–501 (1979).
Bollini R, Van derWilden W, Chrispeels MJ: A precursor of the reserve-protein phaseolin is transiently associated with the endoplasmic reticulum of developing Phaseolus vulgaris cotyledons. Physiol Plant 55: 82–92 (1982).
Bollini R, Vitale A, Chrispeels MJ: In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: evidence for two glycosylation steps. J Cell Biol 96: 999–1007 (1983).
Burnette WN: Western blotting: Electrophoretic transfer of proteins from sodium dodecyl sulfate polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112: 195–203 (1981).
Chou PY, Fasman GD: Predicting the secondary structure of proteins from their amino acid sequence. Adv Enzymol 47: 45–148 (1978).
Chrispeels MJ: The Golgi apparatus mediates the transport of phytohemagglutinin to the protein bodies in bean cotyledons. Planta 158: 140–151 (1983).
Ditta G, Stanfield S, Corbin D, Helinski DR: Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77: 7347–7351 (1980).
Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, Galluppi GR, Goldberry SB, Hoffmann NL, Woo SC: Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA 80: 4803–4807 (1983).
Gething MJ, McCammon K, Sambrook J: Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell 46: 939–950 (1986).
Greenwood JS, Chrispeels MJ: Correct targeting of the bean storage protein phaseolin in the seeds of transformed tobacco. Plant Physiol 79: 65–71 (1985).
Herman EM: Immunocytochemical localization of macromolecules with the electron microscope. Ann Rev Plant Physiol, in press (1988).
Herman EM, Shannon LM: Immunocytochemical evidence for the involvement of Golgi apparatus in the deposition of seed lectin of Bauhinia purpurea (Leguminosae). Protoplasma 121: 163–170 (1984).
Herman EM, Shannon LM: Accumulation and subcellular localization of α-galactosidase hemagglutinin in developing soybean cotyledons. Plant Physiol 77: 886–890 (1985).
Herman EM, Shannon LM, Chrispeels MJ: The Golgi apparatus mediates the transport and post-translational modification of protein body proteins. In: Shannon LM. Chrispeels MJ (eds) Molecular Biology of Seed Storage Proteins and Lectins, pp. 163–173. American Society of Plant Physiologists, Baltimore, MD (1986).
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA: A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180 (1983).
Hoffman LM, Donaldson DD, Brookland R, Rashka K, Herman EM: Synthesis and protein body deposition of maize 15-Kd zein in transgenic tobacco seeds. EMBO J 6: 3213–3221 (1987).
Hoffman LM, Fritsch MK, Gorski J: Probable precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem 256: 2597–2600 (1981).
Horsch RB, Fry JE, Hoffmann NL, Eichholz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1299–1231 (1985).
Hurkman WJ, Beevers L: Sequestration of pea reserve proteins by rough microsomes. Plant Physiol 69: 1414–1417 (1982).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Mains PA, Sibley CH: The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J Biol Chem 258: 5027–5033 (1983).
Melton DA, Kreig PA, Rebagliati MR, Maniatis T, Zinn K, Green MR: Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucl Acids Res 12: 7035–7056 (1984).
Murai N, Sutton DW, Murray MG, Slightom JL, Merlo DJ, Reichert NA, Sengupta-Gopalan C, Stock CA, Barker RF, Kemp JD, Hall TC: Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science 222: 476–482 (1983).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 (1962).
Pederson K, Argos P, Naravana SVL, Larkins BA: Sequence analysis and characterization of a maize gene encoding a high-sulfur zein protein of M r 15000. J Biol Chem 201: 6279–6284 (1986).
Sengupta C, Deluca V, Bailey DS, Verma DPS: Post-translational processing of 7S and 11S components of soybean storage proteins. Plant Mol Biol 1: 19–34 (1981).
Sengupta-Gopalan C, Reichert NA, Barker RF, Hall TC, Kemp JD: Developmentally regulated expression of the bean β-phaseolin gene in tobacco seed. Proc Natl Acad Sci USA 82: 3320–3324 (1985).
Slightom JL, Drong RF, Klassy RC, Hoffman LM: Nucleotide sequences from phaseolin cDNA clones: the major storage proteins from Phaseolus vulgaris are encoded by two unique gene families. Nucl Acids Res 13: 6483–6498 (1985).
Sturm A, VanKuik JA, Vliegenthart JGF, Chrispeels MJ: Structure, position, and biosynthesis of the high mannose and complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem 262: 13392–13403 (1987).
Sun SM, Mutschler MA, Bliss FA, Hall TC: Protein synthesis and accumulation in bean cotyledons during growth. Plant Physiol 61: 918–923 (1978).
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 (1979).
Van derWilden W, Herman EM, Chrispeels MJ: Protein bodies of mung cotyledons as autophagic organelles. Proc Natl Acad Sci USA 77: 428–432 (1980).
Author information
Authors and Affiliations
Additional information
Mention of trademark, proprietary product, or vendor does not constitute a guarantee of warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable. Publication No. 70 from Agrigenetics Advanced Science Company.
Rights and permissions
About this article
Cite this article
Hoffman, L.M., Donaldson, D.D. & Herman, E.M. A modified storage protein is synthesized, processed, and degraded in the seeds of transgenic plants. Plant Mol Biol 11, 717–729 (1988). https://doi.org/10.1007/BF00019513
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019513