Abstract
Coronaviruses are pathogens with a serious impact on human and animal health. They mostly cause enteric or respiratory disease, which can be severe and life threatening, e.g., in the case of the zoonotic coronaviruses causing severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) in humans. Despite the economic and societal impact of such coronavirus infections, and the likelihood of future outbreaks of additional pathogenic coronaviruses, our options to prevent or treat coronavirus infections remain very limited. This highlights the importance of advancing our knowledge on the replication of these viruses and their interactions with the host. Compared to other +RNA viruses, coronaviruses have an exceptionally large genome and employ a complex genome expression strategy. Next to a role in basic virus replication or virus assembly, many of the coronavirus proteins expressed in the infected cell contribute to the coronavirus-host interplay. For example, by interacting with the host cell to create an optimal environment for coronavirus replication, by altering host gene expression or by counteracting the host’s antiviral defenses. These coronavirus–host interactions are key to viral pathogenesis and will ultimately determine the outcome of infection. Due to the complexity of the coronavirus proteome and replication cycle, our knowledge of host factors involved in coronavirus replication is still in an early stage compared to what is known for some other +RNA viruses. This review summarizes our current understanding of coronavirus–host interactions at the level of the infected cell, with special attention for the assembly and function of the viral RNA-synthesising machinery and the evasion of cellular innate immune responses.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Around the end of 2002, an outbreak of a previously unknown severe acute respiratory syndrome (SARS) started in South East China and Hong Kong. Accelerated by air travel, the disease rapidly spread to several parts of the world and displayed pandemic potential. SARS-coronavirus (SARS-CoV) was identified as the causative agent of this zoonotic infection (Drosten et al. 2003; Ksiazek et al. 2003; Kuiken et al. 2003; Peiris et al. 2003), which resulted in >8000 laboratory-confirmed cases and 774 associated deaths worldwide (WHO 2004). Although in terms of death toll not comparable to influenza, HIV or HCV, the 2003 SARS-CoV outbreak caused worldwide public concern and seriously affected the global economy [estimated losses $30–100 billion; (Keogh-Brown and Smith 2008)]. SARS-CoV initially causes lower respiratory tract disease, which can lead to a progressive and potentially lethal atypical pneumonia with clinical symptoms that include fever, malaise, lymphopenia, and in some cases also diarrhea. Two years after the outbreak, horseshoe bats were identified as the likely reservoir of the SARS virus, whereas civet cats probably have served as intermediate host during the zoonotic transfer to humans (Lau et al. 2005; Li et al. 2005b). Adaptation to the human host required a small number of mutations in the receptor-binding domain of the SARS-CoV spike (S) protein, which mediates cell binding and entry (Li et al. 2005c) (see Chap. 2). There is increasing evidence that SARS-like coronaviruses continue to circulate in bats and that these may have the potential to readily cross the species barrier and emerge as human pathogens (Ge et al. 2013; Menachery et al. 2015). Such zoonotic scenarios therefore remain a serious public health concern.
Almost a decade after the SARS-CoV outbreak, the next zoonotic coronavirus emerged: Middle East Respiratory Syndrome coronavirus (MERS-CoV) (de Groot et al. 2013). The virus was first isolated in June 2012 from a 60-year-old Saudi Arabian male who died from acute respiratory distress syndrome (ARDS) and multiple organ failure, including renal failure (Zaki et al. 2012; van Boheemen et al. 2012). Also MERS-CoV can cause a lower respiratory tract infection with symptoms that include coughing and high fever. By the end of 2016, more than 1850 laboratory-confirmed MERS-CoV cases had been recorded, with a mortality rate of about 35% (WHO 2016). MERS-CoV is assumed to be transmitted to humans from camels and serological studies in the latter animals revealed that they have harbored MERS-CoV or MERS-CoV-like viruses for decades (Muller et al. 2014).
Besides the zoonotic SARS- and MERS-CoVs, the coronavirus family includes four ‘established’ human coronaviruses (HCoVs), of which HCoV-OC43 and -229E have already been known since the 1960s. These two viruses cause mild respiratory disease and, after rhinoviruses, are a leading cause of common colds (10–30% of the cases) (van der Hoek 2007; McIntosh et al. 1967; Hamre and Procknow 1966). More recently, following intensified screening for coronaviruses, two additional HCoVs were discovered, HCoV-NL63 (van der Hoek et al. 2004) and HCoV-HKU1 (Woo et al. 2005). Interestingly, recent findings suggest that also HCoV-NL63, -229E, and -OC43 originate from zoonotic transfer from bats (Huynh et al. 2012; Corman et al. 2016; Vijgen et al. 2006; Corman et al. 2015). Coronaviruses also cause a range of infectious diseases in animal species, some with serious (economical) consequences for the livestock industry. This is illustrated by the recent emergence of a novel variant of porcine epidemic diarrhea virus, which is closely related to a strain that caused a large outbreak in China in 2010, killing almost one million piglets [for a recent review, see (Lin et al. 2016)].
The economic impact of coronavirus infections, the past and likely future emergence of pathogenic zoonotic coronaviruses and the lack of effective antiviral strategies have made it painfully clear that our preparedness to treat or prevent coronavirus infections are very limited. This highlights the importance of advancing our knowledge on the replication of these viruses and their interactions with the host.
Coronaviruses are positive-stranded RNA (+RNA) viruses with, for this kind of viruses, exceptionally large genomes of ~30 kb. They have a polycistronic genome organization and employ a unique transcription mechanism to generate a nested set of subgenomic (sg) mRNAs. These are used to express the open reading frames (ORFs) located downstream of the replicase ORFs 1a and 1b (see Fig. 1a), which encode structural and accessory proteins. The sg mRNAs are 3′ co-terminal but they also contain a common 5′ leader sequence. The leader and ‘body’ segments of the sg RNAs are joined during discontinuous negative strand RNA synthesis, which produces a subgenome-length template for each of the sg mRNAs [(Sawicki and Sawicki 1995), for a recent review, see (Sola et al. 2015)].
Outline of the coronavirus replicative cycle and replicase polyprotein organization, based on SARS-CoV. a Schematic overview of the coronavirus replicative cycle. Following entry by receptor-mediated endocytosis and release of the genome into the cytosol, genome translation yields the pp1a and pp1ab replicase polyproteins. Following polyprotein cleavage by multiple internal proteases, the viral nsps assemble into an RTC that engages in minus-strand RNA synthesis. Both full-length and subgenome (sg)-length minus strands are produced, with the latter templating the synthesis of the sg mRNAs required to express the structural and accessory protein genes residing in the 3′-proximal quarter of the genome. Ultimately, novel genomes are packaged into nucleocapsids that become enveloped by budding from smooth intracellular membranes, after which the new virions leave the cell by following the exocytic pathway. See text for more details. b The 14 open reading frames in the genome are indicated, i.e., the replicase ORFs 1a and 1b, the four common CoV structural protein genes (S, E, M, and N) and the ORFs encoding so-called ‘accessory proteins.’ The bottom panel explains the organization and proteolytic processing of the pp1a and pp1ab replicase polyproteins, the latter being produced by −1 ribosomal frameshifting. The nsp3 (PLpro) and nsp5 (3CLpro) proteases and their cleavage sites are indicated in matching colors. The resulting 16 cleavage products [nonstructural proteins (nsps)] are indicated, as are the conserved replicase domains. Domain abbreviations and corresponding nsp numbers: PLpro, papain-like proteinase (nsp3); 3CLpro, 3C-like protease (nsp5); TM, transmembrane domain (nsp3, nsp4, and nsp6); NiRAN, nidovirus RdRp-associated nucleotidyl transferase (nsp12); RdRp, RNA-dependent RNA polymerase (nsp12); ZBD, zinc-binding domain (nsp13); HEL1, superfamily 1 helicase (nsp13); ExoN, exoribonuclease (nsp14); N7-MT, N7-methyl transferase (nsp14); endoU, uridylate-specific endoribonuclease (nsp15); 2′-O-MT, 2′-O-methyl transferase (nsp16). Adopted with permission from (Snijder et al. 2016)
Following entry and uncoating, the coronavirus replicative cycle (see Fig. 1a) starts with the translation of the 5′-proximal ORFs of the viral genome (ORF1a and ORF1b), which results in the synthesis of two large replicase polyproteins (pp1a and pp1ab). Synthesis of pp1ab, a C-terminally extended form of pp1a, involves a -1 ribosomal frameshift (RFS) into ORF1b occurring near the 3′ end of ORF1a. This regulatory mechanism is thought to have evolved to downregulate expression levels of ORF1b-encoded proteins compared to ORF1a-encoded nonstructural proteins (nsps) (Brierley and Dos Ramos 2006; Brierley et al. 1989). Ultimately, 15 or 16 mature replicase proteins are released from pp1a and pp1ab due to proteolytic cleavages performed by two or three ORF1a-encoded proteases. Nsp3 contains one or two papain-like protease domains (PL1pro and PL2pro, or PLpro for SARS-CoV and infectious bronchitis virus) that process the nsp1-4 part of the replicase polyproteins. The remaining cleavage sites are processed by the viral main protease that resides in nsp5, a chymotrypsin-like enzyme also known as 3C-like protease (Snijder et al. 2016). A schematic overview of the proteolytic processing and domain structure of the SARS-CoV replicase is presented in Fig. 1b. The replicase proteins contain a variety of (enzymatic) activities and functions that are required for viral RNA synthesis and capping (Perlman and Netland 2009; Snijder et al. 2016), such as the RNA-dependent RNA polymerase (RdRp; nsp12), a helicase (nsp13), RNA cap-modifying methyltransferases (nsp14 and nsp16), and an exoribonuclease (nsp14). Together with recruited host cell proteins, the coronavirus nsps form membrane-associated replication and transcription complexes [RTCs; (van Hemert et al. 2008)], which localize to a network of virus-induced membrane structures in the perinuclear region of the infected cell (Knoops et al. 2008; Gosert et al. 2002; van der Meer et al. 1999; Brockway et al. 2003; Stertz et al. 2007; Ulasli et al. 2010). Many of the nsps appear to have multiple functions in the synthesis or processing of viral RNA, or in virus–host interactions aiming to create an optimal environment for coronavirus replication, for example by facilitating viral entry, gene expression, RNA synthesis or virus release. Moreover, to further enhance viral replication, host gene expression and antiviral defenses are targeted in several ways. Coronavirus–host interactions also play a decisive role in viral pathogenesis and the ultimate outcome of infection.
Due to the exceptional size of their +RNA genome and proteome, and the resulting complexity of the interactions with the host, our knowledge of host factors involved in coronavirus replication is still in an early stage compared to what is known for some other +RNA virus groups. In this review, we will summarize our current understanding of coronavirus–host interactions at the level of the infected cell, with special attention for the assembly and function of the viral RNA-synthesizing machinery and the evasion of cellular innate immune responses.
2 Host Receptors Involved in Coronavirus Entry
Entry into the target cell constitutes the first critical step in the coronavirus replication cycle. The major determinant for this step is the efficient binding of the coronavirus S glycoprotein to a protein-receptor on the cell surface. The coronavirus S protein is a type 1 glycoprotein that consists of S1 and S2 subunits and is present on the virion surface as a trimer. (Li 2016; Hulswit et al. 2016). The S1 region is involved in receptor binding and contains N- and C-terminal domains (S1-NTD and S1-CTD, respectively) (Walls et al. 2016) that may both act as receptor-binding domain (RBD), with the major determinants of cell tropism residing in S1-CTD. The elongated S2 regions form the stalk of the spike trimer and are mainly involved in triggering the fusion of the viral envelope and target cell membranes [for recent reviews on coronavirus entry and spike protein organization, see (Li 2016; Hulswit et al. 2016)].
The S1-NTD is mainly involved in facilitating virus binding and entry, by interacting with glycans on the host cell surface. Based on the crystal structure of the betacoronavirus S1-NTD and the sequence conservation among the S1-NTDs of other coronaviruses, all coronavirus S1-NTDs are thought to share a galectin fold that mediates binding to sialic acids, like N-glycolylneuraminic acid (Neu5Gc), N-acetylneuraminic acid (Neu5Ac), and/or 5-N-acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2) (see (Li 2016), and references herein). An exception is the murine hepatitis virus (MHV) S1-NTD, which binds the N-terminal D1 domain of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), a type-I membrane protein belonging to the immunoglobulin superfamily (Walls et al. 2016; Williams et al. 1991).
To mediate entry into host cells, the S1-CTD of most known members of the alphacoronavirus genus interacts with aminopeptidase N (APN) (for an overview and references, see Table 1). However, the alphacoronavirus HCoV-NL63 uses a different type-I membrane glycoprotein, angiotensin-converting enzyme 2 (ACE2) (Wu et al. 2009), which contains a large N-terminal ectodomain composed of two alpha-helical lobes. The same molecule, ACE2, has been identified as a receptor for the zoonotic betacoronavirus SARS-CoV (Li et al. 2003). The betacoronaviruses MERS-CoV and bat coronavirus HKU4 use yet another cellular peptidase for virus entry: dipeptidyl peptidase 4 (DPP4) (Yang et al. 2014; Raj et al. 2013). The MERS-CoV S protein has a higher affinity for human DPP4, while the HKU4 S protein binds more strongly to bat DPP4 (Yang et al. 2014). Chemical peptidase inhibitors do not affect virus entry, indicating that SARS-CoV and MERS-CoV receptor usage and entry are independent of the receptor’s peptidase activity and merely depend on binding to these particular host receptors (Li et al. 2005c; Raj et al. 2013).
Besides the receptors discussed above, also extracellular, cell surface-associated and/or lysosomal proteases play a role in coronavirus entry by activating the fusion activity of the S protein [for a recent review, see (Li 2016)]. For SARS-CoV, fusion of the viral and cellular membrane is triggered upon cleavage of the S protein by the cell surface-associated transmembrane protease, serine 2 (TMPRSS2) (Glowacka et al. 2011). The same protease is important for cleavage and activation of the HCoV-229E and MERS-CoV S protein (Shirato et al. 2013; Bertram et al. 2013). After endocytosis, the SARS-CoV S protein is cleaved by the lysosomal proteases cathepsin L and cathepsin P in early endosomes, leading to fusion of the virus envelop with the endosome membranes and release of the viral RNA into the cytosol of the infected cell (Huang et al. 2006a, b; Simmons et al. 2005). MERS-CoV entry occurs by a similar mechanism (Shirato et al. 2013; Burkard et al. 2014), although inhibition of the cellular protease furin abolished the entry of MERS-CoV but not SARS-CoV, indicating that furin-mediated cleavage is pivotal for efficient MERS-CoV entry (Burkard et al. 2014; Follis et al. 2006). On the other hand, MHV strain A59 was shown to fuse with late endosomes and to depend on their low pH for S protein cleavage (Burkard et al. 2014). Therefore, it has been proposed that coronavirus fusion with endosomes depends on the use of a furin cleavage site just upstream of the fusion peptide (Burkard et al. 2014). However, why some coronaviruses fuse with early endosomes and others with late endosomes, and whether these events play a role in host tropism and pathogenicity, is still not completely understood (Burkard et al. 2014). The complexity of S protein cleavage is further highlighted by a recent paper by Park et al., which clearly showed that MERS-CoV entry depends on furin-mediated cleavage in virus-producing cells. Subsequently, cleaved MERS-CoV S proteins could be processed by proteases on recipient cells and virions could enter the cells via early endosomes or even by fusing with the plasma membrane. MERS-CoV virions that contain uncleaved S proteins may rather fuse with late endosomes (Park et al. 2016).
The interaction of the coronavirus S glycoprotein with its cell surface receptor is a key determinant for host tropism. In the case of SARS-CoV, only a few mutations (N479L and T487S) in the S protein’s RBD sufficed to dramatically increase the affinity for human ACE2 (Li 2008). Likewise, the MERS-CoV S protein contains two mutations compared to the bat coronavirus HKU4 S protein, which can bind the human DPP4 receptor, but cannot mediate viral entry due to lack of activation by human proteases. The two mutations in the MERS-CoV S protein (S746R and N762A) enable cleavage by the human proteases and thus viral entry into human cells and may have contributed to the zoonotic transfer of MERS-CoV (Yang et al. 2015).
Several lineage A betacoronaviruses also carry a hemagglutinin-esterase (HE) protein on their surface. HE proteins contain a lectin-binding domain that mediates binding to O-acetylated sialic acids, while also possessing sialate-O-acetylesterase receptor-destroying enzyme activity targeting these same glycans on the cell surface. The receptor-destroying enzyme activity is thought to prevent attachment to non-permissive cells, while the HE protein also facilitates entry into target cells after binding to the main entry receptor (Zeng et al. 2008; Langereis et al. 2010; Bakkers et al. 2016).
3 Translation and the Unfolded Protein Response in Coronavirus-Infected Cells
All viruses depend on the host cell’s translation machinery for the production of their proteins and infectious progeny. Moreover, protein synthesis is also pivotal for the host cell’s response to infection by mounting an antiviral (innate) immune response. Hence, it is not surprising that many +RNA viruses modulate host protein synthesis in order to limit the translation of cellular mRNAs and favor the synthesis of viral proteins [reviewed in (Walsh and Mohr 2011; Fung et al. 2016)]. In eukaryotic cells, translation is initiated by formation of the heterotrimeric eIF2 complex, which is composed of the regulatory α-subunit, the tRNA-binding β-subunit, and a GTP-binding γ-subunit. The eIF2 complex is responsible for loading of the 40S subunit with Met-tRNAi. After mRNA binding, this 43S complex serves as a scaffold for the recruitment of several additional proteins, including eIF3, to the capped 5′ end of the mRNA. Subsequently, the cap-binding eukaryotic translation initiation factor 4F (eIF4F) joins this pre-initiation complex (48S complex), which then scans the mRNA in the 5′ to 3′ direction to localize a translation initiation codon. At this point, the 60S ribosomal subunit joins and protein synthesis starts [reviewed in (Jackson et al. 2010)]. Polyadenine-binding protein (PABP), which binds to the poly(A)-tail of mRNAs, is also involved in stimulating protein synthesis.
The eIF2 complex can be inactivated by phosphorylation of its alpha subunit (eIF2α) by one of four mammalian kinases in response to various (external) triggers. These kinases are eIF2α kinase 4 (also known as GCN2), heme-regulated inhibitor (HRI), PKR-like endoplasmic reticulum kinase (PERK), which is activated upon induction of ER stress, and double-stranded (ds) RNA-activated protein kinase (PKR).
Since several stages of the coronavirus replication cycle are closely associated with the endoplasmic reticulum (ER), ER stress is thought to occur during coronavirus infection. Indeed, expression of several coronavirus proteins, including the heavily glycosylated S protein, was shown to induce ER stress, which was also observed in coronavirus-infected cells [(Chan et al. 2006), and reviewed in (Fung et al. 2016)]. Consequently, the unfolded protein response (UPR) is induced, which alleviates the problems by inhibiting translation (by PERK-induced phosphorylation of eIF2α), stimulating protein folding, and eventually triggering apoptosis. Compared to, for example, hepatitis C virus [see review by (Chan 2014)], many details of how coronaviruses control the UPR remain unknown, but they generally seem to manipulate PERK activity to control the level of translation [reviewed by (Fung et al. 2016)].
PKR is a serine/threonine protein kinase that is activated by the presence of dsRNA, a hallmark of RNA virus infection. PKR is a key player in the innate immune response to RNA virus infection as it upregulates antiviral gene expression, including the production of interferons (IFNs). Coronaviruses have evolved various strategies to counteract PKR-mediated signaling in order to prevent the translational shut-off due to eIF2α phosphorylation. For example, infectious bronchitis virus (IBV) appears to (weakly) antagonize PKR by blocking its activation as well as inducing the expression of growth arrest and DNA-damage-inducible 34 protein (GADD34), leading to reduced eIF2α phosphorylation in IBV-infected cells (Wang et al. 2009). Upon MHV infection, sustained eIF2α phosphorylation and repression of GADD34 expression leads to translational repression of cellular mRNAs, which may be beneficial for MHV infection (Bechill et al. 2008). Recently, the MERS-CoV ORF4a protein was shown to counteract the PKR-induced formation of stress granules, probably by binding viral dsRNA to shield it from detection by PKR, thereby preventing translational inhibition (Rabouw et al. 2016). Also transmissible gastroenteritis virus (TGEV) has been reported to modulate host cell translation, in this case through its protein 7, which promotes eIF2α dephosphorylation through an interaction with protein phosphatase 1 (PP1), a key regulator of the host’s antiviral response (Cruz et al. 2011). The S proteins of both SARS-CoV and IBV were found to physically interact with eIF3F, to modulate host translation, including the expression of the pro-inflammatory cytokines interleukin (IL) 6 and 8, at a later stage of infection (Xiao et al. 2008). Therefore, this interaction may play an important regulatory role in coronavirus pathogenesis.
Besides modulating eIF2α phosphorylation, coronaviruses have other ways of manipulating the translation machinery. Importantly, the nsp1 proteins of both alpha- and betacoronaviruses were identified as inhibitors of multiple steps of translation initiation (Lokugamage et al. 2012, 2015). SARS-CoV nsp1 does so by inhibiting 48S initiation complex formation and interfering with its conversion into the 80S initiation complex (Lokugamage et al. 2012). In addition, the multifunctional SARS-CoV nsp1 is able to directly bind the 40S ribosomal subunit to inhibit its function in translation (Kamitani et al. 2009). Moreover, this complex of nsp1 and the 40S subunit induces cleavage of cellular mRNAs to suppress host cell translation to an even larger extent (Kamitani et al. 2006). MERS-CoV nsp1 seems to act differently, by selectively inhibiting the translation of mRNAs produced in the nucleus, while leaving the translation of the cytosolically made viral mRNAs unaffected (Lokugamage et al. 2015). The difference with SARS-CoV nsp1 is further highlighted by the observation that MERS-CoV nsp1 does not bind to the 40S ribosomal subunit (Lokugamage et al. 2015).
Taken together, several coronavirus studies have highlighted how modulation of host protein synthesis through different—often parallel—mechanisms can have a profound effect on the cell. In this manner, viral ‘translation modulators’ may contribute importantly to coronavirus pathogenicity.
4 Coronavirus-Induced Modification of Host Cell Membranes
As outlined in Chap. 1, a common characteristic of +RNA viruses is that their RNA synthesis takes place in the cytoplasm and is associated with virus-induced structures derived from cellular endomembranes [reviewed in (Romero-Brey and Bartenschlager 2016; Reid et al. 2015; van der Hoeven et al. 2016)]. This is an intriguing kind of virus–host interaction and the architecture of these ‘replication organelles’ has now been studied in detail for quite a number of viruses. Nevertheless, their exact functions have remained largely obscure. In general, two types of +RNA virus-induced membrane structures have been recognized [recently reviewed by (van der Hoeven et al. 2016)]. The first type is characterized by single-membrane spherules, invaginations with a negative curvature formed in the membranes of organelles such as the endoplasmic reticulum (ER), peroxisomes, or endosomes, with the source of the membrane depending on the virus under study. The viral replication machinery is located within these spherules and RNA products are exported through a channel that connects the spherule’s interior and the cytosol, so that they can engage in translation or particle assembly. Flaviviruses and alphaviruses are examples of virus families inducing the formation of this type of replication organelles [reviewed in (den Boon and Ahlquist 2010)].
The second type of replication structures, which includes those found in coronavirus-infected cells, is dominated by double-membrane vesicles (DMVs), often accompanied by other structures such as tubules, zippered ER and/or convoluted membranes, together forming a reticulovesicular network in the cytosol (Knoops et al. 2008; Maier et al. 2013; Ulasli et al. 2010; Hagemeijer et al. 2012; Gosert et al. 2002) (Fig. 1c). Picornaviruses, arteriviruses, and flaviviruses like hepatitis C virus (HCV) induce similar structures [reviewed in (van der Schaar et al. 2016; van der Hoeven et al. 2016; Paul et al. 2014), respectively]. It is generally thought that viral nsps that have transmembrane regions, or are otherwise anchored to membranes, drive the formation of these structures. In the case of coronaviruses, nsp3, nsp4, and nsp6 have been implicated in this process (Angelini et al. 2013; Hagemeijer et al. 2012).
In terms of host factors and pathways involved in the formation of coronavirus replication organelles, a variety of hypotheses and data sets have been put forward. Much of this data is still under debate though, making it quite difficult to formulate a consensus theory. The involvement of ER membranes seems to be generally accepted and is supported by the presence of ribosomes and ER markers such as sec61α and protein disulphide isomerase (PDI) on the surface of or inside virus-induced double-membrane structures, and the fact that the outer membrane of coronavirus-induced DMVs can be continuous with ER cisternae (Hagemeijer et al. 2014; Knoops et al. 2008, 2010; Snijder et al. 2006). In accordance with this link to the ER, studies by de Haan and co-workers (Oostra et al. 2007; Verheije et al. 2008) suggested that the secretory pathway, including coatomer protein (COP)-dependent processes and associated factors such as GBF-1, plays an important role in replication. However, no co-localization was observed between nsp4, a marker for the coronavirus-induced membrane structures, and secretory pathway markers. These observations are in agreement with the results of our own siRNA screen for host factors influencing SARS-CoV replication, which identified COPB2 (or β′-COP), a subunit of the coatomer protein complex, as a strong proviral (or ‘dependency’) factor (de Wilde et al. 2015). Depletion of COPB1 and GBF1, which are part of this same machinery, also severely affected SARS-CoV replication, confirming that the integrity of the secretory pathway is important for replication (de Wilde et al. 2015; Knoops et al. 2010). Several reports have described the importance of phosphatidylinositol 4-kinases (PI4Ks) in +RNA virus replication, which was first discovered through siRNA screens searching for cellular factors important for picornavirus and hepatitis C virus replication (Reiss et al. 2011; Hsu et al. 2010; Berger et al. 2009). These kinases seem to be recruited to the sites of membrane modification and stimulate the production of PI4P lipids, which together supports the formation and/or functionality of viral replication structures. The underlying mechanism is not exactly clear yet, and several hypotheses have been put forward [reviewed in (Altan-Bonnet and Balla 2012)]. Also for SARS-CoV replication one of the PI4K isoforms, PI4KIIIbeta, was shown to be important (Yang et al. 2012), although it seems to play a role in entry rather than later steps of the replication cycle. However, in our kinome-based siRNA screen, PI4Ks were not identified as cellular factors involved in SARS-CoV replication (de Wilde et al. 2015), although siRNA screens are known to yield false-negative results.
A long-standing hypothesis regarding coronavirus-induced double-membrane structures is the possible involvement of the autophagy pathway, which derives from the fact that autophagosomes also have double membranes. Some reports suggested that coronaviruses hijack the autophagy machinery for DMV biogenesis in support of their replication (de Haan and Reggiori 2008; Prentice et al. 2004; Maier and Britton 2012). However, another study showed that the essential autophagy factor Atg5 is not required at all for coronavirus replication in primary cells (Zhao et al. 2007). Molinari and co-workers then proposed that so-called EDEMosomes are being hijacked for the formation of coronavirus membrane structures (Reggiori et al. 2010). These EDEMosomes are defined as single-membrane vesicles that pinch off from the ER to remove ERAD regulators (like EDEM1 and OS-9) when this is needed to tune the ERAD machinery (Cali et al. 2008). The process seems a deviation from the autophagy pathway, with the EDEMosomes accumulating LC3-I, a form that is inactive in the canonical autophagy pathway. Reggiori and co-workers claimed that coronaviruses hijack these vesicles to form their reticulovesicular network, and this hypothesis was later extended to arteriviruses (Monastyrska et al. 2013). However, several questions have remained unanswered and other published data appear to be at odds with the EDEMosome hypothesis. For example, it has remained entirely unclear how the small single-membrane EDEMosome vesicles would be converted into the elaborate network of (much larger) DMVs, convoluted membranes (CM), and other structures that are typical of coronavirus-infected cells. Furthermore, EDEMosomes have been characterized as alternative transport vesicles that explicitly are not associated with COP-coats and are independent of the canonical secretory pathway, which—as Reggiori and co-workers argued—may explain why secretory pathway markers do not localize to replication membranes (Reggiori et al. 2010). Nonetheless, the integrity of the secretory pathway and the function of COP components clearly influences coronavirus replication (Verheije et al. 2008; Oostra et al. 2007; Knoops et al. 2010; de Wilde et al. 2015).
All in all, although a variety of studies addressed the possible host pathways and factors involved in the formation and function of the replication-associated membrane structures, our understanding of coronavirus replication organelle biogenesis is still far from complete. Possibly, the expansion of our basic knowledge regarding relevant cellular processes, such as membrane trafficking and autophagy, may provide more clues on the molecular mechanisms underlying these interesting interactions of coronaviruses with their host cells.
5 Host Proteins Interacting with the Coronavirus Genome and Its Replication or Expression
The 5′- and 3′-proximal regions of coronavirus RNAs contain key regulatory elements for their RNA synthesis [for a recent review, see (Yang and Leibowitz 2015)]. Although in general the precise role of host factors interacting with these signals is poorly understood, RNA-binding proteins have been identified as frequently used enhancers of coronaviral RNA synthesis (Table 2). Both termini of the coronavirus genome fold into higher-order RNA structures, which presumably stabilize the molecule and are also involved in inter- and intramolecular interactions that facilitate viral replication (Brian and Baric 2005). Viral and cellular proteins can bind to these structures to drive or modulate translation, replication, and subgenomic RNA synthesis.
The cellular protein polypyrimidine tract-binding protein (PTB; or heterogeneous ribonucleoprotein protein (hnRNP) I) was found to bind the 5′ leader sequence of the TGEV (Galan et al. 2009) and MHV genome (Li et al. 1999; Choi et al. 2002). In the case of MHV, PTB was found to bind a 5′-proximal pentanucleotide UCUAA repeat and to be critical for RNA synthesis. HnRNP Q, or SYNCRIP, also binds the 5′-proximal part of the MHV genome and its knockdown reduced MHV RNA synthesis and virus replication. The case for a specific role in RNA synthesis was strengthened by the observation that neither overexpression nor downregulation affected translation of MHV RNA (Choi et al. 2004). Zinc finger CCHC-type and RNA-binding motif 1 (MADP1) was shown to bind the 5′ end of the SARS-CoV and IBV genome (Tan et al. 2012). In analogy to SYNCRIP, silencing of MAPD1 reduced IBV replication by interfering with viral RNA synthesis, showing that MAPD1 plays a proviral role in the coronavirus cycle (Tan et al. 2012).
In addition to proteins that bind the 5′ UTR of the coronavirus RNA, many RNA-binding proteins have been identified that interact with the 3′ UTR and/or poly(A)-tail, although the role of these proteins is poorly understood. First, mitochondrial aconitase, stabilized by a complex of heat-shock protein (hsp) 40, hsp60, and mitochondrial hsp70, binds the 3′-terminal 42 nucleotides of the MHV 3′ UTR, just upstream the poly(A) tail (Nanda et al. 2004; Nanda and Leibowitz 2001). The protein p100 coactivator was found to bind the TGEV 3′ UTR and/or poly(A) tail (Galan et al. 2009) and the PABPs interact with the TGEV, BCoV and IBV genome to promote its efficient replication (Spagnolo and Hogue 2000; Galan et al. 2009; Emmott et al. 2013). It has been proposed that binding of PABPs to the viral RNA ensures efficient translation and mRNA stability (Enjuanes 2005). In addition, by using an RNA-affinity purification and mass spectrometry approach, Galan et al. (2009) identified several hnRNPs that bind the 3′-terminal ~500 nucleotides of the TGEV genome. Several of these are presumed RNA-binding proteins for other coronaviruses and were proposed to play a role in viral RNA synthesis (Table 2). However, the exact function and relevance of hnRNPs and other RNA-binding proteins in coronavirus replication is still not fully understood. The majority of RNA-binding proteins were identified in co-immunoprecipitation studies that made use of in vitro transcribed RNA and the importance of many of these factors has not been tested in the context of virus replication. For example, hnRNP A1 was reported to have affinity for the complement of the MHV leader sequence and the TRS that regulates mRNA7 synthesis (Li et al. 1997; Zhang and Lai 1995). Interestingly, however, Shen and Masters (Shen and Masters 2001) showed that MHV replicates equally well in hnRNP A1−/− CB3 cells and parental CB3 cells, which lack and express hnRNP A1, respectively. This strongly suggests that hnRNP A1 itself is not pivotal for MHV replication. On the other hand, Lai and co-workers showed that multiple hnRNPs can bind to the same viral RNA and postulated that hnRNPs may be able to functionally substitute each other in MHV infection (Shi et al. 2003). These apparent contradictions also highlight the technical complexity of dissecting the precise role of RNA-binding proteins in coronavirus replication.
On a different level, RNA-binding proteins were investigated as potential modulators of the efficiency of the coronavirus ORF1a/1b frameshift event during replicase gene translation. For example, annexin A2 binds the IBV slippery sequence, on which ribosomes are repositioned and engage in expression of ORF1b. Host proteins may also affect the activity of the viral RTC indirectly. Using an in vitro assay, the RNA-synthesizing activity of semi-purified SARS-CoV RTCs was shown to depend on an as yet unidentified cytosolic host factor, which is not directly associated with the viral RTC (van Hemert et al. 2008).
6 Host Innate Immune Responses Against Coronaviruses, and Viral Countermeasures
Cells generally respond to a virus infection by mounting an innate antiviral response to limit the spread of the infection and aid in inducing an adaptive immune response that will eventually clear the virus. Many viruses have evolved strategies to suppress and/or evade these (innate) immune responses, which can dramatically influence the course of the infection, including pathogenesis and persistence in the host. In the case of +RNA viruses, the innate immune system is often triggered by the dsRNA and 5′-triphosphate-bearing RNA molecules that arise as replication intermediates in the cytosol. These molecules are foreign to the cell and can be recognized by the intracellular sensors of the Rig-I-like receptor (RLR) family, such as retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5) which are expressed in almost all cells [reviewed in (Wilkins and Gale 2010; Bruns and Horvath 2014)]. For recognition of coronavirus RNAs, MDA-5 seems the most important cytosolic sensor (Zust et al. 2011; Zalinger et al. 2015; Kindler and Thiel 2014), although in some cell types RIG-I also seems to play a role (Li et al. 2010). Also the toll-like receptors which are expressed on the cell surface or reside in the endosomes of immune cells can recognize viral nucleic acids or proteins. TLR3 plays a role in the recognition of coronaviruses (Totura et al. 2015; Mazaleuskaya et al. 2012) and also TLR4 was shown to be relevant during MHV infection in mice (Khanolkar et al. 2009). Recently it was shown that the SARS-CoV M protein is recognized via a TLR-like pathway that is independent of the canonical TRAF3-mediated signaling pathway (Wang and Liu 2016). Activation of one or more of these sensors generally leads to the activation of the transcription factors IFN-regulatory factor 3 and 7 (IRF3, IRF7) and NF-κB. These stimulate the expression and excretion of Type-I IFN and pro-inflammatory cytokines, which in turn activate the JAK-STAT signaling cascade that induces the expression of a myriad of antiviral interferon-stimulated genes (ISGs). This ultimately results in an antiviral state of the infected cell, as well as neighboring cells. ISGs were shown to target virtually all steps of the viral cycle in order to restrict viral replication (Schoggins and Rice 2011). The p38 mitogen-activated protein kinases (MAPKs) play a role in the induction of inflammatory cytokines IL-6 and IL-8, and were linked to countering coronavirus infections through several studies [reviewed in (Mizutani 2007)]. IBV has evolved a strategy to counteract IL-6 and IL-8 expression by inducing the expression of dual-specificity phosphatase 1 (DUSP1), a negative regulator of p38 MAPK, although it has remained unclear which viral protein(s) is responsible (Liao et al. 2011). Innate immune and inflammatory signaling pathways are extensively regulated in order to prevent adverse effects of their over-stimulation. Apart from phosphorylation and other regulatory mechanisms, the system is controlled by ubiquitination at numerous points in the signal transduction cascade. For example, RIG-I, TANK-binding kinase 1 (TBK1), and TNF receptor-associated factor 3 (TRAF3) were shown to be activated by Lys63-linked ubiquitination (Jiang and Chen 2012).
The importance of the innate immune system in the context of coronavirus infections can be illustrated in at least three ways. First, in severe cases of SARS the pathology was associated with aberrant or hyper-activation of innate immune signaling. This resulted in the aberrant production of interferons and high levels of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, CXCL-10 and TNF-alpha in the lungs [reviewed in (Totura and Baric 2012)]. Interestingly, a systems biology study that evaluated the transcriptome after infection of cultured cells with two different human MERS-CoV isolates (MERS-CoV Eng 1 vs. SA 1) suggested that viral sequence differences relate to variations in innate immune evasion, which may in turn result in different immune responses. These differences may link to differential STAT3 activation leading to activation or inhibition of, e.g., IFN, NF-κB, and IRF7 (Selinger et al. 2014). Second, as described in Chap. 4, coronaviruses employ elaborate mechanisms to shield the viral replication machinery from the innate immune sensors in the cytosol. Third, besides the presumed shielding of viral PAMPs by the replication organelles, coronaviruses seem to encode numerous gene products that actively counteract or help to circumvent innate immune responses [reviewed in (Totura and Baric 2012; Kindler and Thiel 2014; Vijay and Perlman 2016)].
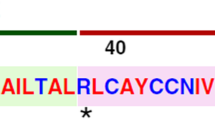
To illustrate the multitude of activities coronaviruses employ to actively suppress innate immunity, and the diversity of viral gene products involved, some examples are discussed below. First, besides inhibiting cellular mRNA translation (see paragraph above), SARS-CoV nsp1 was shown to block IFN signaling by reducing the amount of phosphorylated STAT1 (p-STAT1) in infected cells (Wathelet et al. 2007). Also nsp1 proteins of other alpha- and betacoronaviruses were shown to inhibit type-I IFN signaling, mostly through the host-shut-off activity of this N-terminal subunit of the coronavirus replicase polyprotein (reviewed in Narayanan et al. 2015). Further downstream in the pp1a polyprotein, the papain-like protease 2 domain (PL2pro) of many coronaviruses and the TGEV PL1pro domain, which reside in nsp3, exhibit deubiquitination (DUB) activity in biochemical experiments [reviewed in (Mielech et al. 2014)]. This DUB activity may remove ubiquitin from innate immune signaling factors to suppress the induction of an antiviral state, and indeed was shown to reduce IFN signaling in biochemical experiments using PL2pro overexpression for several coronaviruses, including SARS-CoV (Matthews et al. 2014a; Li et al. 2016) and MERS-CoV (Chen et al. 2007; Clementz et al. 2010; Bailey-Elkin et al. 2014). Similarly, experiments suggested that MHV PL2pro deubiquitinates and binds TBK1, as well as IRF3 (Zheng et al. 2008; Wang et al. 2011). For the distantly related arterivirus EAV, infection with a mutant lacking a similar papain-like protease-driven DUB activity resulted in an increased innate immune response after infection, indicating that the viral DUB activity indeed has a function in suppression of innate immune response during infection (van Kasteren et al. 2013). Adjacent to the PL2pro domain, some coronaviruses contain a domain originally coined ‘SARS-CoV unique domain’ (SUD). The two domains together were recently also implicated in innate immune suppression by binding and stimulating a cellular ubiquitin E3 ligase, RCHY1, resulting in augmented degradation of p53 (Ma-Lauer et al. 2016). The main protease, nsp5, of PEDV was shown to cleave NEMO, an important innate immune regulator protein (Wang et al. 2015). When independently expressed, coronavirus nsp6, which is probably one of the most hydrophobic proteins encoded in the genome, seems to induce and/or influence autophagy. However the relevance of this observation for virus-infected cells and possible links to innate immune responses against coronaviruses remain to be investigated (Cottam et al. 2014; Cottam et al. 2011). A conserved domain in the coronavirus nsp16 directs 2′-O methylation of the viral RNA, thereby preventing its recognition by MDA5 (Menachery et al. 2014; Zust et al. 2011).
Besides conserved replicase subunits, also several of the less-conserved products of downstream open reading frames, i.e., the so-called ‘accessory proteins’, have innate immunity suppressing features. In fact, these proteins may have been acquired by different coronavirus lineages for this purpose, since they are generally dispensable for replication in cell culture. The phosphodiesterase (ns2) encoded by ORF2a in MHV and related viruses antagonizes RNAse L activation, and this was show to be important for replication in natural host cells (Zhao et al. 2012; Li and Weiss 2016). Observations supporting a role in blocking IFN production and/or signaling were also made for the ORF3b and ORF6 proteins of SARS-CoV (Kopecky-Bromberg et al. 2007) and the ORF3b proteins of other SARS-like coronaviruses (Zhou et al. 2012). Also the proteins encoded by MERS-CoV ORFs 4a, 4b and 5 (Siu et al. 2014; Yang et al. 2013; Niemeyer et al. 2013; Matthews et al. 2014b) all seem to block IFN signaling, although many of the initial reports were based only on overexpression experiments, which should be interpreted with caution. Recently however, more insight into the mechanisms by which some of these viral proteins suppress antiviral responses was obtained. Like MHV ns2 (see above), the MERS-CoV ORF4b protein and its homologs from other betacoronaviruses were shown to have phosphodiesterase activity. They can block activation of the RNAse L-mediated innate immune response during infection by degrading 2′,5′-oligoadenylate, which is the activator of RNAse L (Thornbrough et al. 2016). Additionally, MERS-CoV ORF4a prevents activation of the PKR-stress granule route by binding dsRNA, in the context of an infection (Rabouw et al. 2016). The SARS-CoV ORF6 protein was shown to block p-STAT1 import into the nucleus by interacting with the nuclear pore, and this block was suggested to reduce innate immune responses and the expression of genes that affect virus-induced pathogenesis (Frieman et al. 2007; Kopecky-Bromberg et al. 2007; Huang et al. 2015a). Interestingly, MERS-CoV, along with the other coronaviruses not belonging to the SARS-CoV cluster, lacks an ORF6 homolog, and since MERS-CoV is also more sensitive to treatment with type-I interferons, it was hypothesized that this could—in part—be explained by this difference (de Wilde et al. 2013b). Also the coronaviral structural proteins seem to possess innate immunity-modulating activities, although also here most studies involved overexpression experiments. The SARS-CoV and MERS-CoV M proteins bind TRAF3 to prevent its binding to TBK1 and eventually to prevent nuclear translocation of this complex, which blocks IRF3-mediated signaling (Siu et al. 2009; Lui et al. 2016). Overexpression experiments showed a negative impact of the SARS-CoV N protein on (the early stages of) innate immune signaling (Frieman et al. 2009; Kopecky-Bromberg et al. 2007; Lu et al. 2011). MHV N protein also seems to counteract type-I IFN signaling, and could do this in the context of a recombinant vaccinia virus infection (Ye et al. 2007). Purified SARS-CoV spike (S) protein stimulates inflammatory and other innate responses, possibly through TLR2 activation (Dosch et al. 2009). Finally, the SARS-CoV E protein, is a viroporin, and influences inflammatory processes by boosting the activity of the NLRP3 inflammasome, leading to IL-1beta overproduction and development of immunopathology in the host (Nieto-Torres et al. 2015).
7 Coronavirus-Induced Deregulation of the Cell Cycle
The cell cycle is a series of highly regulated events that leads to cell division. The process can be divided into four distinct phases: G1, S, G2, and M. Cell cycle regulation is critical for cell survival, as well as the prevention of uncontrolled cell division. The molecular mechanisms that control the cell cycle are ordered, directional, and controlled by cyclin-dependent protein kinases (CDKs). Cell cycle progression requires activation of different CDKs by, e.g., cyclin regulatory subunits. To reach the stage of DNA replication, CDK/cyclin complexes phosphorylate, and thereby activate or inactivate, their target proteins to coordinate progression towards the next phase of the cell cycle (Nigg 1995).
Like many other viruses [reviewed in (Bagga and Bouchard 2014)], coronaviruses have been shown to extensively manipulate and arrest cell cycle progression, to benefit from the physiological state of cells arrested in that specific phase. For example, IBV-infected cells were shown to go into cell cycle arrest in the S phase, by activating the cellular DNA damage response (Xu et al. 2011). This is beneficial to virus replication since factors that are normally needed for DNA replication and are upregulated in the S phase, can now be recruited to the cytoplasm by the virus. For example, DDX1, a cellular RNA helicase of the DExD/H family, interacts with coronavirus nsp14 (Xu et al. 2010) and was reported to be hijacked by coronaviruses to enhance their replication. DDX1 also interacts with the IBV N protein (Emmott et al. 2013) and facilitates, in complex with the phosphorylated form of the MHV-JHM N protein, the balanced synthesis of sg mRNAs and the genomic RNA (Wu et al. 2014).
Bhardwaj et al. have shown that coronavirus nsp15 interacts with and inhibits retinoblastoma protein (pRb), a tumor suppressor protein. This results in the enhanced expression of genes that are normally repressed by pRb and in an increased proportion of cells entering the S phase of the cell cycle (Bhardwaj et al. 2012). Similar effects have been observed in MHV-infected cells, which showed decreased hyperphosphorylation of pRb, an event that is necessary for the progression from G1 to S phase (Chen and Makino 2004; Chen et al. 2004). Yuan and colleagues showed that overexpression of the SARS-CoV 3a protein also leads to G1 arrest and inhibition of cell proliferation (Yuan et al. 2007).
The cyclin-dependent kinase 6 (CDK6) is downregulated upon MHV infection and seems to play a role in a virus-induced cell cycle arrest in the G0/G1 phase that promotes virus replication (Chen and Makino 2004). Similar observations have been made for SARS-CoV, with (overexpression of) the N protein limiting cell cycle progression by reducing CDK4 and CDK6 kinase activity (Surjit et al. 2006). CDK6 is a kinase involved in cell cycle progression from G1 to S phase (Jimenez-Guardeno et al. 2014) and depletion of CDK6 results in G1 phase cell cycle arrest. A host kinome-directed siRNA screen confirmed the antiviral role of CDK6 in SARS-CoV infection, as replication was enhanced in cells depleted for CDK6 (de Wilde et al. 2015).
Several coronaviruses induce activation of p53 to mediate cell cycle arrest in the S or G2/M phase. For example, TGEV N protein activates p53 which leads to accumulation of cell cycle-related kinases, like cdc-2 and cyclin B1. Synchronisation of cells in the S or G2/M phase favors TGEV RNA and virus production (Ding et al. 2013, 2014) and IBV replication (Dove et al. 2006). For MHV nsp1 a similar mechanism has been proposed: nsp1 activates p53, leading to increased p21 and decreased CDK2-cyclin E levels. This ultimately leads to hyperphosphorylation of pRb and G1 cell cycle arrest (Chen et al. 2004). In contrast, SARS-CoV infection leads to reduction of p53 expression levels. To counteract the inhibitory effect of p53, its expression is reduced by a mechanism that involves stabilization of the E3 ligase RCHY1 by SARS-CoV nsp3. RCHY1 mediates the ubiquitination and degradation of p53. However, the exact role of this mechanism in cell cycle regulation, viral replication and/or pathogenesis remains unclear (Ma-Lauer et al. 2016). Nevertheless, in cells that lack p53 expression, SARS-CoV replication was significantly enhanced (Ma-Lauer et al. 2016). Degradation of p53 by the SARS-CoV or HCoV-NL63 PL2pro is another mechanism to counteract the antiviral effect of p53. The deubiquitinating activity of PL2pro promotes the degradation of p53, thereby lowering the p53-mediated antiviral immune response (Yuan et al. 2015).
In conclusion, coronaviruses appear to favor a specific stage in the cell cycle for their replication. This stage can differ per coronavirus and may even differ per cell type. A wide variety of coronavirus proteins have been implicated in inducing cell cycle arrest, but it must be noted that many studies involved overexpression of individual viral proteins and therefore should be interpreted with caution since their expression levels are likely different than during virus infection. When expressed outside the context of virus replication, these proteins may also behave differently (e.g., due to lack of viral interaction partners) and/or localize differently (e.g., in the absence of virus-induced membrane structures), which might lead to less meaningful observations.
8 The Role of Cyclophilins in Coronavirus Replication
Cyclosporin A (CsA) is a well-known immunosuppressant that binds to cellular cyclophilins (Cyps), yielding a Cyp-CsA complex that inhibits calcineurin activity. This in turn prevents dephosphorylation and translocation of nuclear factor of activated T cells (NF-AT) from the cytosol into the nucleus, which prevents the transcription of immune genes, such as IL-2 [reviewed in (Tanaka et al. 2013; Davis et al. 2010; Barik 2006)]. Thus far, 17 Cyps have been identified, of which nine are targeted by CsA. Cyps are also known as peptidyl–prolyl isomerases (PPIases) and many of them have chaperone and foldase activities (Barik 2006; Davis et al. 2010) that facilitate protein folding. Cyps are involved in various signaling pathways [reviewed in (Barik 2006)], apoptosis (Schinzel et al. 2005), and RNA splicing (Teigelkamp et al. 1998; Horowitz et al. 2002).
Cyclophilins, and in particular the cytosolic CypA and the ER-associated CypB, have been implicated in the replicative cycle of many RNA viruses as essential host components [reviewed in (Baugh and Gallay 2012)]. For example, (i) Cyps are essential in the remodeling of cellular membranes into HCV replication organelles, (ii) CypA aids in HCV polyprotein processing, (iii) HIV-1 capsids are stabilized by low levels of CypA to ensure entrance into the nucleus before the virion could be destabilized in the cytosol [(Hopkins and Gallay 2015), and references herein]. The use of CsA analogs, like Alisporivir (Paeshuyse et al. 2006), that lack the immunosuppressive properties of the parental compound, has been explored in clinical trials for the treatment of chronic HCV infection, again illustrating the prominent role of Cyps in HCV replication and the druggability of Cyps (Flisiak et al. 2012).
Along the same lines, the inhibition of coronavirus replication by Cyp inhibitors like CsA and Alisporivir suggested important roles for Cyps. In cell culture infection models, low-micromolar concentrations of CsA or Alisporivir inhibit a variety of coronaviruses, including SARS-CoV, HCoV-229E, MHV, HCoV-NL63, and FCoV (Pfefferle et al. 2011; de Wilde et al. 2011; Tanaka et al. 2012; Carbajo-Lozoya et al. 2012; de Wilde et al. 2017). The mitochondrial CypD is part of the mitochondrial permeabilization transition pore and involved in caspase-independent apoptosis induced by porcine epidemic diarrea virus (PEDV) and HCoV-NL63 (Favreau et al. 2012; Kim and Lee 2014), an event that is inhibited by CsA. However, most studies on the presumed role of Cyps in coronavirus replication have focused on CypA. Initially, CypA was identified as an interaction partner of the SARS-CoV N protein (Luo et al. 2004) and by mass spectrometry it was also detected in purified SARS-CoV virions (Neuman et al. 2008). Yeast two-hybrid experiments identified multiple Cyps (CypA, CypB, CypH, and CypG) and related PPIases (FK506-binding protein 1A and 1B) as potential binding partners of SARS-CoV nsp1 (Pfefferle et al. 2011). In addition, in Caco-2 or Huh7 cells, shRNA-mediated knockdown of CypA expression to less than 3% of the normal levels was reported to near-completely block HCoV-NL63 replication and reduce HCoV-229E replication by >1 log (Carbajo-Lozoya et al. 2014; von Brunn et al. 2015). Recently, by using PPIA or PPIB knockout rather than knockdown cells, it was shown that FCoV depends on both CypA and CypB expression (>95% reduction in FCoV-infected CypA or CypB-KO cells). PPIase-deficient mutants, expressed in cells that also contained endogenous CypA and CypB, marginally reduced FCoV infection (two- to fivefold reduction in FCoV RNA compared to cells that express wt CypA or wt CypB only) (Tanaka et al. 2016). Furthermore, the work of von Brunn and colleagues suggested that HCoV-229E replication was reduced by various CypA single-nucleotide polymorphisms that affect the protein’s stability and function (von Brunn et al. 2015). Although these results suggested a role for CypA in coronavirus replication, the siRNA-mediated depletion of CypA and CypB did not affect the replication of SARS-CoV (de Wilde et al. 2011), despite the fact that the same knockdown did affect the replication of the distantly related arterivirus EAV (de Wilde et al. 2013a). The differences reported using either knockout or knockdown of CypA with different efficiencies (100%, >97%, and ~75% for CypA knockout cells, and CypA shRNA- or siRNA-mediated knockdown, respectively) suggest that low CypA expression levels may suffice to support efficient coronavirus replication and that the (near-)complete depletion of CypA may be needed to inhibit virus replication.
The major role of Cyps in cellular signaling is in the NF-AT signaling pathway. Pfefferle et al. described that SARS-CoV activates the NF-AT signaling pathway (Pfefferle et al. 2011) and the replication of various coronaviruses, including SARS-CoV, HCoV-229E, and HCoV-NL63, is inhibited by the drug FK-506, which—like CsA—also blocks NF-AT signaling (Carbajo-Lozoya et al. 2012). Feline coronavirus replication seemed not to depend on a functional NF-AT signaling pathway since it was blocked by CsA concentrations that did not affect NF-AT signaling in fcwf-4 cells (Tanaka et al. 2012).
In conclusion, further studies, are needed to dissect the precise role of Cyps in coronavirus replication, with special attention for the (remaining) levels of CypA expression, and the relevance of NF-AT signaling following coronavirus infection. Such experiments should include the production and use of knockout cells for one or multiple Cyps or other members of this protein family. Although Cyp inhibitors have been shown to be potent anti-coronavirus drugs in cell culture, a first attempt to validate this effect for Alisporivir in a SARS-CoV animal model was unsuccessful (de Wilde et al. 2017). Therefore, although Cyp inhibitors may help to understand the role of host factors in coronavirus replication, they are at this moment less promising as host-directed therapeutics for the treatment of coronavirus infections.
9 Systems Biology Approaches to Identifying Host Factors in Coronavirus Replication
The application of systems biology approaches in virology has provided a wealth of information on the role of individual proteins and cellular pathways in the replication of RNA viruses. This relatively young, interdisciplinary field focuses on the complexity of the virus–host interactions that occur within the cell or even the whole organism. The aim is to provide an unbiased perspective, by applying techniques like transcriptomics, metabolomics, proteomics, and functional genomics to the infected system as a whole.
For coronaviruses, one of the first systematic studies into the role of host factors concerned an oligonucleotide microarray-based transcriptomic analysis of SARS-CoV-infected peripheral blood mononuclear cells, which revealed the upregulation of the expression of various cytokines, including IL-8 and IL-17, and the activation of macrophages and the coagulation pathway (Ng et al. 2004). A microarray analysis of lung autopsy tissue samples provided more insight into the pathogenesis of and host response to SARS-CoV infection, in particular the inflammatory and cytokine responses involved (Baas et al. 2006). MHV-JHM infected cultures of central nervous system cells showed 126 differentially expressed transcripts, the majority of which were related to intracellular regulation of innate immunity (e.g., NF-κB signaling and genes involved in IFN signaling) (Rempel et al. 2005). Microarray analysis of MHV-A59-infected L cells provided insight into transcriptional changes during infection, including those related to chemokine production, RNA and protein metabolism and apoptosis (Versteeg et al. 2006). Subsequently, a genome-wide microarray analysis of MHV-infected LR7 cells revealed the downregulation of a large number of mRNAs, including many encoding proteins involved in translation, implying that the host translational shut-off that occurs in MHV-infected cells is due to a stress response and concomitant mRNA decay (Raaben et al. 2007). There is not necessarily a direct correlation between changes in mRNA levels and protein abundance in the cell. Therefore, microarray data should be interpreted with caution and need to be validated with follow-up experiments including the direct analysis of changes in cellular protein levels.
Another approach to obtain more insight into coronavirus–host interactions is to systematically map the cellular interactome of individual coronavirus proteins. A yeast two-hybrid (Y2H) screen of this type identified subunits (BTF3 and ATF5) of the RNA polymerase complex and a subunit of cytochrome oxidase II (NADH 4L) as interaction partners of SARS-CoV nsp10 (Li et al. 2005a). The authors suggested the latter interaction to contribute to the SARS-CoV-induced cytopathic effect, but the interaction and its relevance for virus-induced cell death still awaits confirmation in the context of SARS-CoV-infected cells, as the rather artificial Y2H system is known to frequently yield false-positive hits. Indeed, another Y2H study with the SARS-CoV helicase (nsp13) demonstrated that out of the seven primary hits only one, DDX5, could be validated by independent methods as a true interactor of the helicase nsp13 (Chen et al. 2009). The functional significance of the interaction between nsp13 and the multifunctional cellular helicase DDX5, and whether the interaction is direct or mediated through RNA, remains to be determined. Using a similar Y2H screening approach, Xu and colleagues found that DDX1 bound to SARS-CoV and IBV nsp14, an interaction that enhanced IBV replication. The presence of one or more cellular helicases in the viral RTC supports their importance in virus replication. Indeed a later study reported that DDX1, regulated by the GSK-3-mediated phosphorylation of the N protein, is involved in the regulation of sg mRNA synthesis (Wu et al. 2014). A very comprehensive systematic analysis of the SARS-CoV-host interactome was performed by Pfefferle et al. using a high-throughput genome-wide Y2H screen with all 14 SARS-CoV ORFs and fragments thereof (Pfefferle et al. 2011). Network analysis revealed particularly striking interactions between nsp1 and the members of the immunophilin family, including CypA (see Chap. 8). Furthermore, the cellular E3 ubiquitin ligase RCHY1 was shown to interact with the SARS-CoV nsp3 SUD domain and might be involved in the downregulation of the antiviral factor p53 (Ma-Lauer et al. 2016).
Stable isotope labeling with amino acids in cell culture (SILAC) is a mass spectrometry-based proteomics technique to determine differences in protein abundance between samples from two different experimental conditions, e.g., comparing virus-infected with uninfected cells. The first SILAC-based quantitative proteomics study of infected cells demonstrated the upregulation of NF-κB and AP-1 dependent pathways during IBV infection (Emmott et al. 2010). A combination of SILAC on 293T cells that express the IBV N protein, pull-down and mass spectrometry was used to map the cellular interactome of the IBV N protein, leading to the identification of 142 cellular proteins as potential binding partners (Emmott et al. 2013). Many of these proteins are interacting with RNA, e.g., ribosomal and nucleolar proteins, helicases, and hnRNPs (Emmott et al. 2013) and therefore likely bind the IBV N protein indirectly. Nevertheless, detailed validation and mechanistic follow-up studies confirmed the functional importance of several of the identified binding partners for IBV replication (Emmott et al. 2013).
The Baric laboratory used a systems genetics approach using the Collaborative Cross mouse panel to gain insight into the host loci that affect the outcome of SARS-CoV infection (Gralinski et al. 2015). This study—among other findings—identified the ubiquitin E3 ligase Trim55 as an important determinant of disease severity through its role in vascular cuffing and inflammation. A study by Selinger et al. (2014) documented differences in immune and inflammatory responses in MERS patients, which may codetermine the outcome of the infection and likely result from both differences in host response (genetic make-up) and MERS-CoV strain-specific properties. To better understand the molecular basis of the different immune and inflammatory responses to two different MERS-CoV isolates, a comparative transcriptome analysis was done on human airway cells infected with MERS-CoV strains SA1 and Eng1 (Selinger et al. 2014). This study suggested that differences in genome replication and/or proteins involved in innate immune evasion (PLpro and ORF4a) were responsible for different transcriptional responses, resulting in the differential activation of the STAT3 pathway, which is likely involved in lung inflammation and cellular repair. These effects are mainly seen during later stages of infection, and with the MERS-CoV Eng1 strain triggering a more rapid host response than the SA1 strain.
A SILAC-based quantitative proteomics study that compared the proteome of SARS-CoV replicon-expressing BHK-21 cells with that of control cells identified 43 host proteins whose expression was upregulated and 31 that were downregulated (Zhang et al. 2010). BAG3, a multifunctional regulator of many cellular processes was identified as one of the upregulated proteins and knockdown studies revealed that BAG3 is important for efficient replication of SARS-CoV and a number of other viruses. In addition, many proteins involved in translation and the signaling proteins Cdc42 and RhoA were shown to be downregulated, as discussed in more detail in Sect. 3 (Zhang et al. 2010). A SILAC-based quantitative proteomics study of Golgi-enriched subcellular fractions revealed that upon MHV infection several proteins of the secretory pathway were depleted, while ribosomal proteins were found to be enriched (Vogels et al. 2011). SiRNA-mediated knockdown of three of the depleted proteins, C11orf59, GLG1, and sec22b, increased replication or release of infectious progeny, while overexpression of these proteins had the opposite effect. This study highlighted the importance of the secretory pathway in coronavirus replication.
A role for secretory pathway proteins was also confirmed by a proteomic analysis of purified SARS-CoV virions, which identified, besides several viral proteins including nsp2, nsp3, and nsp5, 172 host proteins in virions (Neuman et al. 2008). Several proteins from the COPI pathway were identified, which is in line with the site of virion biogenesis (ERGIC). The role, if any, of most of the identified host proteins in SARS-CoV replication or virion biogenesis remains to be elucidated.
Protein kinases are key regulators in signal transduction, control a wide variety of cellular processes, and have been shown to play important roles in the replicative cycle of many +RNA viruses. A kinome-wide siRNA screen identified a variety of host cell kinases that influence SARS-CoV replication, including 40 ‘proviral’ proteins that promote efficient replication (de Wilde et al. 2015). Among these, proteins involved in the metabolism of complex lipids and the early secretory pathway (COPI-coated vesicles) were found to play an important role. The antiviral effect of PKR was confirmed in this study and CDK6 was identified as a novel antiviral factor. A relatively large number of antiviral hits (90 of 778 factors; ~12% of all factors tested) was identified for SARS-CoV compared to human kinome-directed screens performed with other viruses (Supekova et al. 2008; Lupberger et al. 2011; Moser et al. 2010). This might indicate that, compared to other viruses, SARS-CoV replication is more extensively influenced by cellular factors. Multiple of these factors could be linked to cellular immune responses, like interleukin (IL) signaling, which (IL-6 and -8) was also implicated in controlling coronavirus infection and coronavirus-induced inflammation in other studies (Zhang et al. 2007; Baas et al. 2006). Also several proteins from the p38 MAPK pathway were identified, which had also been implicated in coronavirus replication earlier and regulates IL-6-, IL-8-, and IL-10-mediated pro-inflammatory cytokine signaling (Chang et al. 2004; Zhang et al. 2007; Song et al. 2013). The interaction between coronaviruses and the innate immune response was already discussed in more detail in Chap. 6. A similar, genome-wide, siRNA screen identified host proteins important for the replication of IBV (Wong et al. 2015), including 83 proviral proteins, 30 of which could be mapped to networks that interact with viral proteins. Many of the identified proteins are involved in RNA binding/processing, membrane trafficking and ubiquitin conjugation. The importance of the secretory pathway that was demonstrated by de Wilde et al. (2015) was in line with an earlier study that demonstrated that MHV replication was sensitive to Brefeldin A treatment and dependent on GBF1-mediated ARF1 activation, which appear to be involved in RTC formation (Verheije et al. 2008). Similar results were also obtained for IBV (Wong et al. 2015) and this study also identified an early role in IBV infection for the valosin-containing protein (VCP) which may be involved in the maturation of virus-loaded endosomes. VCP is also important for the early stages of HCoV-229E replication (Wong et al. 2015). An RNAi screen of the druggable genome identified several endocytosis-related proteins that are required for efficient infection of HeLa cells with MHV (Burkard et al. 2014). Subsequent validation and mechanistic studies, demonstrated that—as discussed above—clathrin-mediated endocytosis and trafficking to lysosomes are crucial for MHV fusion and entry, which required the activity of lysosomal proteases. This is different for MERS-CoV, which contains a furin cleavage site upstream of the fusion peptide in the Spike protein, and therefore requires furin activity, but not lysosomal proteases (Burkard et al. 2014).
RNAi screens have revolutionized functional genetics, but major concerns are the possibility of false-positive hits due to off-target effects (including downregulation of multiple transcripts), stimulation of the immune response, or saturation of the RNAi machinery leading to a block in processing of essential cellular (mi)RNAs [reviewed in (Jackson and Linsley 2010)]. Insufficient knockdown of host factors can lead to false-negative results. Therefore, hits from siRNA screens need to be thoroughly validated, preferably using an independent technical approach. They should be considered as a mere starting point for further analysis rather than providing a definitive list of host factors involved in virus replication. Technological advancements and novel screening approaches, e.g, those based on CRISPR/Cas9-mediated genome editing or haploid genetic screens (Shalem et al. 2014; Carette et al. 2009), will likely lead to the more reliable identification of host factors important for coronavirus replication.
10 Concluding Remarks
Insight into coronavirus-host interactions, obtained, e.g., using systematic screening approaches, does not only yield valuable information on the molecular details of the replicative cycle and pathogenesis, but can also be a starting point for the development of antiviral strategies. Virus binding and entry are the first steps of the replication cycle that can be targeted with inhibitors. Several well-known inhibitors of endosomal acidification, like ammonium chloride and the FDA-approved anti-malaria drug chloroquine, have been shown to block entry of coronaviruses (Takano et al. 2013; Keyaerts et al. 2009; Krzystyniak and Dupuy 1984; Payne et al. 1990; Kono et al. 2008), including SARS-CoV and MERS-CoV (Keyaerts et al. 2004; de Wilde et al. 2014). In addition, peptides have been developed that block fusion by interfering with the interaction between the HR1 and HR2 domains of the S protein, preventing the formation of a fusogenic complex or blocking S protein oligomerisation [reviewed in (Du et al. 2009)].
Interferon (IFN) was shown to trigger the innate immune response in coronavirus-infected cells, leading to transcription of many ISGs that have a role in controlling infection (Schoggins and Rice 2011). Treatment with type-I IFNs inhibits coronavirus replication in cell culture (Garlinghouse et al. 1984; Taguchi and Siddell 1985; Haagmans et al. 2004; Paragas et al. 2005; Zheng et al. 2004; de Wilde et al. 2013b) and, for example, protected type-I pneumocytes against SARS-CoV infection in macaques (Haagmans et al. 2004). Despite the potency of IFN as an antiviral agent, its side effects like fatigue, malaise, apathy, and cognitive changes (Dusheiko 1997) emphasize the need for developing IFN-free therapeutic strategies. Besides inhibitors directed at viral enzymes (Kim et al. 2016), such therapeutic strategies could also involve host-directed approaches, based on the knowledge obtained on coronavirus–host interactions. The host-directed approach might lower the chance of development of antiviral resistance and could yield a broad-spectrum therapeutic strategy to treat infections with currently problematic coronaviruses and new variants that will undoubtedly emerge in the future.
References
Altan-Bonnet N, Balla T (2012) Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem Sci 37:293–302
Angelini MM, Akhlaghpour M, Neuman BW, Buchmeier MJ (2013) Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio 4:e00524–e00513
Baas T, Taubenberger JK, Chong PY, Chui P, Katze MG (2006) SARS-CoV virus-host interactions and comparative etiologies of acute respiratory distress syndrome as determined by transcriptional and cytokine profiling of formalin-fixed paraffin-embedded tissues. J Interferon Cytokine Res: The Official Journal of The International Society for Interferon and Cytokine Research 26:309–317
Bagga S, Bouchard MJ (2014) Cell cycle regulation during viral infection. Methods Mol Biol 1170:165–227
Bailey-Elkin BA, Knaap RC, Johnson GG, Dalebout TJ, Ninaber DK, van Kasteren PB, Bredenbeek PJ, Snijder EJ, Kikkert M, Mark BL (2014) Crystal structure of the Middle East respiratory syndrome coronavirus (MERS-CoV) papain-like protease bound to ubiquitin facilitates targeted disruption of deubiquitinating activity to demonstrate its role in innate immune suppression. J Biol Chem 289:34667–34682
Bakkers MJ, Zeng Q, Feitsma LJ, Hulswit RJ, Li Z, Westerbeke A, van Kuppeveld FJ, Boons GJ, Langereis MA, Huizinga EG, de Groot RJ (2016) Coronavirus receptor switch explained from the stereochemistry of protein-carbohydrate interactions and a single mutation. Proc Natl Acad Sci U S A 113:E3111–E3119
Barik S (2006) Immunophilins: for the love of proteins. Cell Mol Life Sci 63:2889–2900
Baugh J, Gallay P (2012) Cyclophilin involvement in the replication of hepatitis C virus and other viruses. Biol Chem 393:579–587
Bechill J, Chen Z, Brewer JW, Baker SC (2008) Coronavirus infection modulates the unfolded protein response and mediates sustained translational repression. J Virol 82:4492–4501
Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G (2009) Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci U S A 106:7577–7582
Bertram S, Dijkman R, Habjan M, Heurich A, Gierer S, Glowacka I, Welsch K, Winkler M, Schneider H, Hofmann-Winkler H, Thiel V, Pohlmann S (2013) TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J Virol 87:6150–6160
Bhardwaj K, Liu P, Leibowitz JL, Kao CC (2012) The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J Virol 86:4294–4304
Brian DA, Baric RS (2005) Coronavirus genome structure and replication. Curr Top Microbiol Immunol 287:1–30
Brierley I, Dos Ramos FJ (2006) Programmed ribosomal frameshifting in HIV-1 and the SARS-CoV. Virus Res 119:29–42
Brierley I, Digard P, Inglis SC (1989) Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell 57:537–547
Brockway SM, Clay CT, Lu XT, Denison MR (2003) Characterization of the expression, intracellular localization, and replication complex association of the putative mouse hepatitis virus RNA-dependent RNA polymerase. J Virol 77:10515–10527
Bruns AM, Horvath CM (2014) Antiviral RNA recognition and assembly by RLR family innate immune sensors. Cytokine Growth Factor Rev 25:507–512
Burkard C, Verheije MH, Wicht O, van Kasteren SI, van Kuppeveld FJ, Haagmans BL, Pelkmans L, Rottier PJ, Bosch BJ, de Haan CA (2014) Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog 10:e1004502
Cali T, Galli C, Olivari S, Molinari M (2008) Segregation and rapid turnover of EDEM1 by an autophagy-like mechanism modulates standard ERAD and folding activities. Biochem Biophys Res Commun 371:405–410
Carbajo-Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A (2012) Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res 165:112–117
Carbajo-Lozoya J, Ma-Lauer Y, Malesevic M, Theuerkorn M, Kahlert V, Prell E, von Brunn B, Muth D, Baumert TF, Drosten C, Fischer G, von Brunn A (2014) Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including alisporivir. Virus Res 184C:44–53
Carette JE, Guimaraes CP, Varadarajan M, Park AS, Wuethrich I, Godarova A, Kotecki M, Cochran BH, Spooner E, Ploegh HL, Brummelkamp TR (2009) Haploid genetic screens in human cells identify host factors used by pathogens. Science 326:1231–1235
Chan SW (2014) Unfolded protein response in hepatitis C virus infection. Front Microbiol 5:233
Chan CP, Siu KL, Chin KT, Yuen KY, Zheng B, Jin DY (2006) Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J Virol 80:9279–9287
Chang YJ, Liu CY, Chiang BL, Chao YC, Chen CC (2004) Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J Immunol 173:7602–7614
Chen CJ, Makino S (2004) Murine coronavirus replication induces cell cycle arrest in G0/G1 phase. J Virol 78:5658–5669
Chen CJ, Sugiyama K, Kubo H, Huang C, Makino S (2004) Murine coronavirus nonstructural protein p28 arrests cell cycle in G0/G1 phase. J Virol 78:10410–10419
Chen Z, Wang Y, Ratia K, Mesecar AD, Wilkinson KD, Baker SC (2007) Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63. J Virol 81:6007–6018
Chen JY, Chen WN, Poon KM, Zheng BJ, Lin X, Wang YX, Wen YM (2009) Interaction between SARS-CoV helicase and a multifunctional cellular protein (Ddx5) revealed by yeast and mammalian cell two-hybrid systems. Arch Virol 154:507–512
Choi KS, Huang P, Lai MM (2002) Polypyrimidine-tract-binding protein affects transcription but not translation of mouse hepatitis virus RNA. Virology 303:58–68
Choi KS, Mizutani A, Lai MM (2004) SYNCRIP, a member of the heterogeneous nuclear ribonucleoprotein family, is involved in mouse hepatitis virus RNA synthesis. J Virol 78:13153–13162
Clementz MA, Chen Z, Banach BS, Wang Y, Sun L, Ratia K, Baez-Santos YM, Wang J, Takayama J, Ghosh AK, Li K, Mesecar AD, Baker SC (2010) Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J Virol 84:4619–4629
Corman VM, Baldwin HJ, Tateno AF, Zerbinati RM, Annan A, Owusu M, Nkrumah EE, Maganga GD, Oppong S, Adu-Sarkodie Y, Vallo P, da Silva Filho LV, Leroy EM, Thiel V, van der Hoek L, Poon LL, Tschapka M, Drosten C, Drexler JF (2015) Evidence for an ancestral association of human coronavirus 229E with bats. J Virol 89:11858–11870
Corman VM, Eckerle I, Memish ZA, Liljander AM, Dijkman R, Jonsdottir H, Juma Ngeiywa KJ, Kamau E, Younan M, Al Masri M, Assiri A, Gluecks I, Musa BE, Meyer B, Muller MA, Hilali M, Bornstein S, Wernery U, Thiel V, Jores J, Drexler JF, Drosten C (2016) Link of a ubiquitous human coronavirus to dromedary camels. Proc Natl Acad Sci U S A 113:9864–9869
Cottam EM, Maier HJ, Manifava M, Vaux LC, Chandra-Schoenfelder P, Gerner W, Britton P, Ktistakis NT, Wileman T (2011) Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy 7:1335–1347
Cottam EM, Whelband MC, Wileman T (2014) Coronavirus NSP6 restricts autophagosome expansion. Autophagy 10:1426–1441
Cruz JL, Sola I, Becares M, Alberca B, Plana J, Enjuanes L, Zuniga S (2011) Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog 7:e1002090
Davis TL, Walker JR, Campagna-Slater V, Finerty PJ, Paramanathan R, Bernstein G, MacKenzie F, Tempel W, Ouyang H, Lee WH, Eisenmesser EZ, Dhe-Paganon S (2010) Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol 8:e1000439
de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J (2013) Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the coronavirus study group. J Virol 87:7790–7792
de Haan CA, Reggiori F (2008) Are nidoviruses hijacking the autophagy machinery? Autophagy 4:276–279
de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, Thiel V, Narayanan K, Makino S, Snijder EJ, van Hemert MJ (2011) Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 92:2542–2548
de Wilde AH, Li Y, van der Meer Y, Vuagniaux G, Lysek R, Fang Y, Snijder EJ, van Hemert MJ (2013a) Cyclophilin inhibitors block arterivirus replication by interfering with viral RNA synthesis. J Virol 87:1454–1464
de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens RW, Posthuma CC, van der Meer Y, Barcena M, Haagmans BL, Snijder EJ, van den Hoogen BG (2013b) MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J Gen Virol 94:1749–1760
de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, van den Hoogen BG, Neyts J, Snijder EJ (2014) Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 58:4875–4884
de Wilde AH, Wannee KF, Scholte FE, Goeman JJ, Ten Dijke P, Snijder EJ, Kikkert M, van Hemert MJ (2015) A kinome-wide siRNA screen identifies proviral and antiviral host factors in SARS-coronavirus replication, including PKR and early secretory pathway proteins. J Virol 89:8318–8333
de Wilde AH, Falzarano D, Zevenhoven-Dobbe JC, Beugeling C, Fett C, Martellaro C, Posthuma CC, Feldmann H, Perlman S, Snijder EJ (2017) Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res 228:7–13
Delmas B, Gelfi J, L’Haridon R, Vogel LK, Sjostrom H, Noren O, Laude H (1992) Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357:417–420
den Boon JA, Ahlquist P (2010) Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol 64:241–256
Ding L, Huang Y, Dai M, Zhao X, Du Q, Dong F, Wang L, Huo R, Zhang W, Xu X, Tong D (2013) Transmissible gastroenteritis virus infection induces cell cycle arrest at S and G2/M phases via p53-dependent pathway. Virus Res 178:241–251
Ding L, Huang Y, Du Q, Dong F, Zhao X, Zhang W, Xu X, Tong D (2014) TGEV nucleocapsid protein induces cell cycle arrest and apoptosis through activation of p53 signaling. Biochem Biophys Res Commun 445:497–503
Dosch SF, Mahajan SD, Collins AR (2009) SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res 142:19–27
Dove B, Brooks G, Bicknell K, Wurm T, Hiscox JA (2006) Cell cycle perturbations induced by infection with the coronavirus infectious bronchitis virus and their effect on virus replication. J Virol 80:4147–4156
Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976
Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S (2009) The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol 7:226–236
Dusheiko G (1997) Side effects of alpha interferon in chronic hepatitis C. Hepatology 26:112S–121S
Emmott E, Rodgers MA, Macdonald A, McCrory S, Ajuh P, Hiscox JA (2010) Quantitative proteomics using stable isotope labeling with amino acids in cell culture reveals changes in the cytoplasmic, nuclear, and nucleolar proteomes in Vero cells infected with the coronavirus infectious bronchitis virus. Mol Cell Proteomics 9:1920–1936
Emmott E, Munday D, Bickerton E, Britton P, Rodgers MA, Whitehouse A, Zhou EM, Hiscox JA (2013) The cellular interactome of the coronavirus infectious bronchitis virus nucleocapsid protein and functional implications for virus biology. J Virol 87:9486–9500
Enjuanes L (2005) Coronavirus replication and reverse genetics. Current Topics in Microbiology and Immunology, vol 287. Springer. doi:10.1007/b138038
Favreau DJ, Meessen-Pinard M, Desforges M, Talbot PJ (2012) Human coronavirus-induced neuronal programmed cell death is cyclophilin d dependent and potentially caspase dispensable. J Virol 86:81–93
Flisiak R, Jaroszewicz J, Flisiak I, Lapinski T (2012) Update on alisporivir in treatment of viral hepatitis C. Expert Opin Investig Drugs 21:375–382
Follis KE, York J, Nunberg JH (2006) Furin cleavage of the SARS coronavirus spike glycoprotein enhances cell-cell fusion but does not affect virion entry. Virology 350:358–369
Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS (2007) Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol 81:9812–9824
Frieman M, Ratia K, Johnston RE, Mesecar AD, Baric RS (2009) Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J Virol 83:6689–6705
Fung TS, Liao Y, Liu DX (2016) Regulation of stress responses and translational control by coronavirus. Viruses 8:184
Furuya T, Lai MM (1993) Three different cellular proteins bind to complementary sites on the 5′-end-positive and 3′-end-negative strands of mouse hepatitis virus RNA. J Virol 67:7215–7222
Galan C, Sola I, Nogales A, Thomas B, Akoulitchev A, Enjuanes L, Almazan F (2009) Host cell proteins interacting with the 3′ end of TGEV coronavirus genome influence virus replication. Virology 391:304–314
Garlinghouse LE Jr, Smith AL, Holford T (1984) The biological relationship of mouse hepatitis virus (MHV) strains and interferon: in vitro induction and sensitivities. Arch Virol 82:19–29
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538
Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pohlmann S (2011) Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 85:4122–4134
Gosert R, Kanjanahaluethai A, Egger D, Bienz K, Baker SC (2002) RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J Virol 76:3697–3708
Gralinski LE, Ferris MT, Aylor DL, Whitmore AC, Green R, Frieman MB, Deming D, Menachery VD, Miller DR, Buus RJ, Bell TA, Churchill GA, Threadgill DW, Katze MG, McMillan L, Valdar W, Heise MT, Pardo-Manuel de Villena F, Baric RS (2015) Genome wide identification of SARS-CoV susceptibility loci using the collaborative cross. PLoS Genet 11:e1005504
Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan KH, Tashiro M, Osterhaus AD (2004) Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 10:290–293
Hagemeijer MC, Rottier PJ, de Haan CA (2012) Biogenesis and dynamics of the coronavirus replicative structures. Viruses 4:3245–3269
Hagemeijer MC, Monastyrska I, Griffith J, van der Sluijs P, Voortman J, van Bergen en Henegouwen PM, Vonk AM, Rottier PJ, Reggiori F, de Haan CA (2014) Membrane rearrangements mediated by coronavirus nonstructural proteins 3 and 4. Virology 458–459:125–135
Hamre D, Procknow JJ (1966) A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med 121:190–193
Hopkins S, Gallay PA (2015) The role of immunophilins in viral infection. Biochim Biophys Acta 1850:2103–2110
Horowitz DS, Lee EJ, Mabon SA, Misteli T (2002) A cyclophilin functions in pre-mRNA splicing. EMBO J 21:470–480
Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, Cameron CE, Ehrenfeld E, van Kuppeveld FJ, Altan-Bonnet N (2010) Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141:799–811
Huang P, Lai MM (1999) Polypyrimidine tract-binding protein binds to the complementary strand of the mouse hepatitis virus 3′ untranslated region, thereby altering RNA conformation. J Virol 73:9110–9116
Huang P, Lai MM (2001) Heterogeneous nuclear ribonucleoprotein a1 binds to the 3′-untranslated region and mediates potential 5′-3′-end cross talks of mouse hepatitis virus RNA. J Virol 75:5009–5017
Huang IC, Bosch BJ, Li F, Li W, Lee KH, Ghiran S, Vasilieva N, Dermody TS, Harrison SC, Dormitzer PR, Farzan M, Rottier PJ, Choe H (2006a) SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J Biol Chem 281:3198–3203
Huang IC, Bosch BJ, Li W, Farzan M, Rottier PM, Choe H (2006b) SARS-CoV, but not HCoV-NL63, utilizes cathepsins to infect cells: viral entry. Adv Exp Med Biol 581:335–338
Huang SH, Lee TY, Lin YJ, Wan L, Lai CH, Lin CW (2015a) Phage display technique identifies the interaction of severe acute respiratory syndrome coronavirus open reading frame 6 protein with nuclear pore complex interacting protein NPIPB3 in modulating type I interferon antagonism. J Microbiol Immunol Infect. doi:10.1016/j.jmii.2015.07.002
Huang X, Dong W, Milewska A, Golda A, Qi Y, Zhu QK, Marasco WA, Baric RS, Sims AC, Pyrc K, Li W, Sui J (2015b) Human coronavirus HKU1 spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J Virol 89:7202–7213
Hulswit RJ, de Haan CA, Bosch BJ (2016) Coronavirus spike protein and tropism changes. Adv Virus Res 96:29–57
Huynh J, Li S, Yount B, Smith A, Sturges L, Olsen JC, Nagel J, Johnson JB, Agnihothram S, Gates JE, Frieman MB, Baric RS, Donaldson EF (2012) Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol 86:12816–12825
Jackson AL, Linsley PS (2010) Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9:57–67
Jackson RJ, Hellen CU, Pestova TV (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11:113–127
Jiang X, Chen ZJ (2012) The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol 12:35–48
Jimenez-Guardeno JM, Nieto-Torres JL, DeDiego ML, Regla-Nava JA, Fernandez-Delgado R, Castano-Rodriguez C, Enjuanes L (2014) The PDZ-binding motif of severe acute respiratory syndrome coronavirus envelope protein is a determinant of viral pathogenesis. PLoS Pathog 10:e1004320
Kamitani W, Narayanan K, Huang C, Lokugamage K, Ikegami T, Ito N, Kubo H, Makino S (2006) Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc Natl Acad Sci USA 103:12885–12890
Kamitani W, Huang C, Narayanan K, Lokugamage KG, Makino S (2009) A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat Struct Mol Biol 16:1134–1140
Keogh-Brown MR, Smith RD (2008) The economic impact of SARS: how does the reality match the predictions? Health Policy 88:110–120
Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M (2004) In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 323:264–268
Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, Maes P (2009) Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother 53:3416–3421
Khanolkar A, Hartwig SM, Haag BA, Meyerholz DK, Harty JT, Varga SM (2009) Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J Virol 83:8946–8956
Kim Y, Lee C (2014) Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology 460–461:180–193
Kim Y, Liu H, Galasiti Kankanamalage AC, Weerasekara S, Hua DH, Groutas WC, Chang KO, Pedersen NC (2016) Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog 12:e1005531
Kindler E, Thiel V (2014) To sense or not to sense viral RNA–essentials of coronavirus innate immune evasion. Curr Opin Microbiol 20:69–75
Knoops K, Kikkert M, van den Worm SH, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, Mommaas AM, Snijder EJ (2008) SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol 6:e226
Knoops K, Swett-Tapia C, van den Worm SH, te Velthuis AJ, Koster AJ, Mommaas AM, Snijder EJ, Kikkert M (2010) Integrity of the early secretory pathway promotes, but is not required for, severe acute respiratory syndrome coronavirus RNA synthesis and virus-induced remodeling of endoplasmic reticulum membranes. J Virol 84:833–846
Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H (2008) Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res 77:150–152
Kopecky-Bromberg SA, Martinez-Sobrido L, Frieman M, Baric RA, Palese P (2007) Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol 81:548–557
Krempl C, Schultze B, Herrler G (1995) Analysis of cellular receptors for human coronavirus OC43. Adv Exp Med Biol 380:371–374
Krzystyniak K, Dupuy JM (1984) Entry of mouse hepatitis virus 3 into cells. J Gen Virol 65:227–231
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, Group SW (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966
Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stohr K, Peiris JS, Osterhaus AD (2003) Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263–270
Kwak H, Park MW, Jeong S (2011) Annexin A2 binds RNA and reduces the frameshifting efficiency of infectious bronchitis virus. PLoS ONE 6:e24067
Langereis MA, van Vliet AL, Boot W, de Groot RJ (2010) Attachment of mouse hepatitis virus to O-acetylated sialic acid is mediated by hemagglutinin-esterase and not by the spike protein. J Virol 84:8970–8974
Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, Wong SS, Leung SY, Chan KH, Yuen KY (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA 102:14040–14045
Li F (2008) Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections. J Virol 82:6984–6991
Li F (2016) Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 3:237–261
Li Y, Weiss SR (2016) Antagonism of RNase L Is required for murine coronavirus replication in kupffer cells and liver sinusoidal endothelial cells but not in hepatocytes. J Virol 90:9826–9832
Li HP, Zhang X, Duncan R, Comai L, Lai MM (1997) Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc Natl Acad Sci USA 94:9544–9549
Li HP, Huang P, Park S, Lai MM (1999) Polypyrimidine tract-binding protein binds to the leader RNA of mouse hepatitis virus and serves as a regulator of viral transcription. J Virol 73:772–777
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454
Li Q, Wang L, Dong C, Che Y, Jiang L, Liu L, Zhao H, Liao Y, Sheng Y, Dong S, Ma S (2005a) The interaction of the SARS coronavirus non-structural protein 10 with the cellular oxido-reductase system causes an extensive cytopathic effect. J Clin Virol: The Official Publication of the Pan American Society for Clinical Virology 34:133–139
Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF (2005b) Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679
Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N, Murakami A, He Y, Marasco WA, Guan Y, Choe H, Farzan M (2005c) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24:1634–1643
Li BX, Ge JW, Li YJ (2007) Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology 365:166–172
Li J, Liu Y, Zhang X (2010) Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J Virol 84:6472–6482
Li SW, Wang CY, Jou YJ, Huang SH, Hsiao LH, Wan L, Lin YJ, Kung SH, Lin CW (2016) SARS coronavirus papain-like protease inhibits the TLR7 signaling pathway through removing Lys63-linked polyubiquitination of TRAF3 and TRAF6. Int J Mol Sci 17:678
Liao Y, Wang X, Huang M, Tam JP, Liu DX (2011) Regulation of the p38 mitogen-activated protein kinase and dual-specificity phosphatase 1 feedback loop modulates the induction of interleukin 6 and 8 in cells infected with coronavirus infectious bronchitis virus. Virology 420:106–116
Lin CM, Saif LJ, Marthaler D, Wang Q (2016) Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res 226:20–39
Liu C, Tang J, Ma Y, Liang X, Yang Y, Peng G, Qi Q, Jiang S, Li J, Du L, Li F (2015) Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J Virol 89:6121–6125
Lokugamage KG, Narayanan K, Huang C, Makino S (2012) SARS coronavirus nsp1 protein is a novel eukaryotic translation inhibitor that represses multiple steps of translation initiation. J Virol 86:13598–13608
Lokugamage KG, Narayanan K, Nakagawa K, Terasaki K, Ramirez SI, Tseng CT, Makino S (2015) Middle East respiratory syndrome coronavirus nsp1 inhibits host gene expression by selectively targeting mRNAs transcribed in the nucleus while sparing mRNAs of cytoplasmic origin. J Virol 89:10970–10981
Lu X, Pan J, Tao J, Guo D (2011) SARS-CoV nucleocapsid protein antagonizes IFN-beta response by targeting initial step of IFN-beta induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes 42:37–45
Lui PY, Wong LY, Fung CL, Siu KL, Yeung ML, Yuen KS, Chan CP, Woo PC, Yuen KY, Jin DY (2016) Middle East respiratory syndrome coronavirus M protein suppresses type I interferon expression through the inhibition of TBK1-dependent phosphorylation of IRF3. Emerg Microbes Infect 5:e39
Luo C, Luo H, Zheng S, Gui C, Yue L, Yu C, Sun T, He P, Chen J, Shen J, Luo X, Li Y, Liu H, Bai D, Yang Y, Li F, Zuo J, Hilgenfeld R, Pei G, Chen K, Shen X, Jiang H (2004) Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem Biophys Res Commun 321:557–565
Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF (2011) EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med 17:589–595
Maier HJ, Britton P (2012) Involvement of autophagy in coronavirus replication. Viruses 4:3440–3451
Maier HJ, Hawes PC, Cottam EM, Mantell J, Verkade P, Monaghan P, Wileman T, Britton P (2013) Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes. MBio 4:e00801–e00813
Ma-Lauer Y, Carbajo-Lozoya J, Hein MY, Muller MA, Deng W, Lei J, Meyer B, Kusov Y, von Brunn B, Bairad DR, Hunten S, Drosten C, Hermeking H, Leonhardt H, Mann M, Hilgenfeld R, von Brunn A (2016) p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci USA 113:E5192–E5201
Matthews K, Schafer A, Pham A, Frieman M (2014a) The SARS coronavirus papain like protease can inhibit IRF3 at a post activation step that requires deubiquitination activity. Virol J 11:209
Matthews KL, Coleman CM, van der Meer Y, Snijder EJ, Frieman MB (2014b) The ORF4b-encoded accessory proteins of Middle East respiratory syndrome coronavirus and two related bat coronaviruses localize to the nucleus and inhibit innate immune signalling. J Gen Virol 95:874–882
Mazaleuskaya L, Veltrop R, Ikpeze N, Martin-Garcia J, Navas-Martin S (2012) Protective role of toll-like receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses 4:901–923
McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM (1967) Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA 57:933–940
Menachery VD, Yount BL Jr, Josset L, Gralinski LE, Scobey T, Agnihothram S, Katze MG, Baric RS (2014) Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2′-o-methyltransferase activity. J Virol 88:4251–4264
Menachery VD, Yount BL Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS (2015) A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21:1508–1513
Mielech AM, Chen Y, Mesecar AD, Baker SC (2014) Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities. Virus Res 194:184–190
Mizutani T (2007) Signal transduction in SARS-CoV-infected cells. Ann N Y Acad Sci 1102:86–95
Monastyrska I, Ulasli M, Rottier PJ, Guan JL, Reggiori F, de Haan CA (2013) An autophagy-independent role for LC3 in equine arteritis virus replication. Autophagy 9:164–174
Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S (2010) A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog 6:e1000954
Muller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, Bosch BJ, Lattwein E, Hilali M, Musa BE, Bornstein S, Drosten C (2014) MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis 20:2093–2095
Nanda SK, Leibowitz JL (2001) Mitochondrial aconitase binds to the 3′ untranslated region of the mouse hepatitis virus genome. J Virol 75:3352–3362
Nanda SK, Johnson RF, Liu Q, Leibowitz JL (2004) Mitochondrial HSP70, HSP40, and HSP60 bind to the 3′ untranslated region of the murine hepatitis virus genome. Arch Virol 149:93–111
Narayanan K, Ramirez SI, Lokugamage KG, Makino S (2015) Coronavirus nonstructural protein 1: common and distinct functions in the regulation of host and viral gene expression. Virus Res 202:89–100
Neuman BW, Joseph JS, Saikatendu KS, Serrano P, Chatterjee A, Johnson MA, Liao L, Klaus JP, Yates JR 3rd, Wuthrich K, Stevens RC, Buchmeier MJ, Kuhn P (2008) Proteomics analysis unravels the functional repertoire of coronavirus nonstructural protein 3. J Virol 82:5279–5294
Ng LF, Hibberd ML, Ooi EE, Tang KF, Neo SY, Tan J, Murthy KR, Vega VB, Chia JM, Liu ET, Ren EC (2004) A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis 4:34
Niemeyer D, Zillinger T, Muth D, Zielecki F, Horvath G, Suliman T, Barchet W, Weber F, Drosten C, Muller MA (2013) Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J Virol 87:12489–12495
Nieto-Torres JL, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Castano-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L (2015) Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 485:330–339
Nigg EA (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 17:471–480
Oostra M, te Lintelo EG, Deijs M, Verheije MH, Rottier PJ, de Haan CA (2007) Localization and membrane topology of coronavirus nonstructural protein 4: involvement of the early secretory pathway in replication. J Virol 81:12323–12336
Paeshuyse J, Kaul A, De Clercq E, Rosenwirth B, Dumont JM, Scalfaro P, Bartenschlager R, Neyts J (2006) The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology 43:761–770
Paragas J, Blatt LM, Hartmann C, Huggins JW, Endy TP (2005) Interferon alfacon1 is an inhibitor of SARS-corona virus in cell-based models. Antiviral Res 66:99–102
Park JE, Li K, Barlan A, Fehr AR, Perlman S, McCray PB Jr, Gallagher T (2016) Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc Natl Acad Sci USA 113:12262–12267
Paul D, Madan V, Bartenschlager R (2014) Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host Microbe 16:569–579
Payne HR, Storz J, Henk WG (1990) Initial events in bovine coronavirus infection: analysis through immunogold probes and lysosomotropic inhibitors. Arch Virol 114:175–189
Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY, group Ss (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325
Perlman S, Netland J (2009) Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7:439–450
Pfefferle S, Schopf J, Kogl M, Friedel CC, Muller MA, Carbajo-Lozoya J, Stellberger T, von Dall’armi E, Herzog P, Kallies S, Niemeyer D, Ditt V, Kuri T, Zust R, Pumpor K, Hilgenfeld R, Schwarz F, Zimmer R, Steffen I, Weber F, Thiel V, Herrler G, Thiel HJ, Schwegmann-Wessels C, Pohlmann S, Haas J, Drosten C, von Brunn A (2011) The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog 7:e1002331
Prentice E, Jerome WG, Yoshimori T, Mizushima N, Denison MR (2004) Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem 279:10136–10141
Raaben M, Groot Koerkamp MJ, Rottier PJ, de Haan CA (2007) Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell Microbiol 9:2218–2229
Rabouw HH, Langereis MA, Knaap RC, Dalebout TJ, Canton J, Sola I, Enjuanes L, Bredenbeek PJ, Kikkert M, de Groot RJ, van Kuppeveld FJ (2016) Middle East respiratory coronavirus accessory protein 4a inhibits PKR-mediated antiviral stress responses. PLoS Pathog 12:e1005982
Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254
Reggiori F, Monastyrska I, Verheije MH, Cali T, Ulasli M, Bianchi S, Bernasconi R, de Haan CA, Molinari M (2010) Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe 7:500–508
Reid CR, Airo AM, Hobman TC (2015) The virus-host interplay: biogenesis of +RNA replication complexes. Viruses 7:4385–4413
Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Buhler S, Pepperkok R, Lengauer T, Albrecht M, Eils R, Schirmacher P, Lohmann V, Bartenschlager R (2011) Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9:32–45
Rempel JD, Quina LA, Blakely-Gonzales PK, Buchmeier MJ, Gruol DL (2005) Viral induction of central nervous system innate immune responses. J Virol 79:4369–4381
Romero-Brey I, Bartenschlager R (2016) Endoplasmic reticulum: the favorite intracellular niche for viral replication and assembly. Viruses 8:E160
Sawicki SG, Sawicki DL (1995) Coronaviruses use discontinuous extension for synthesis of subgenome-length negative strands. Adv Exp Med Biol 380:499–506
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010
Schoggins JW, Rice CM (2011) Interferon-stimulated genes and their antiviral effector functions. Curr opin virol 1:519–525
Schultze B, Herrler G (1992) Bovine coronavirus uses N-acetyl-9-O-acetylneuraminic acid as a receptor determinant to initiate the infection of cultured cells. J Gen Virol 73(Pt 4):901–906
Schultze B, Enjuanes L, Cavanagh D, Herrler G (1993) N-acetylneuraminic acid plays a critical role for the haemagglutinating activity of avian infectious bronchitis virus and porcine transmissible gastroenteritis virus. Adv Exp Med Biol 342:305–310
Schultze B, Krempl C, Ballesteros ML, Shaw L, Schauer R, Enjuanes L, Herrler G (1996) Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J Virol 70:5634–5637
Selinger C, Tisoncik-Go J, Menachery VD, Agnihothram S, Law GL, Chang J, Kelly SM, Sova P, Baric RS, Katze MG (2014) Cytokine systems approach demonstrates differences in innate and pro-inflammatory host responses between genetically distinct MERS-CoV isolates. BMC Genom 15:1161
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87
Shen X, Masters PS (2001) Evaluation of the role of heterogeneous nuclear ribonucleoprotein A1 as a host factor in murine coronavirus discontinuous transcription and genome replication. Proc Natl Acad Sci USA 98:2717–2722
Shi ST, Huang P, Li HP, Lai MM (2000) Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J 19:4701–4711
Shi ST, Yu GY, Lai MM (2003) Multiple type A/B heterogeneous nuclear ribonucleoproteins (hnRNPs) can replace hnRNP A1 in mouse hepatitis virus RNA synthesis. J Virol 77:10584–10593
Shirato K, Kawase M, Matsuyama S (2013) Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 87:12552–12561
Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P (2005) Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A 102:11876–11881
Siu KL, Kok KH, Ng MH, Poon VK, Yuen KY, Zheng BJ, Jin DY (2009) Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J Biol Chem 284:16202–16209
Siu KL, Yeung ML, Kok KH, Yuen KS, Kew C, Lui PY, Chan CP, Tse H, Woo PC, Yuen KY, Jin DY (2014) Middle east respiratory syndrome coronavirus 4a protein is a double-stranded RNA-binding protein that suppresses PACT-induced activation of RIG-I and MDA5 in the innate antiviral response. J Virol 88:4866–4876
Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, Mommaas AM (2006) Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol 80:5927–5940
Snijder EJ, Decroly E, Ziebuhr J (2016) The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv Virus Res 96:59–126
Sola I, Almazan F, Zuniga S, Enjuanes L (2015) Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol 2:265–288
Song S, Bi J, Wang D, Fang L, Zhang L, Li F, Chen H, Xiao S (2013) Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-kappaB and p38 MAPK pathways in porcine alveolar macrophages. Dev Comp Immunol 39:265–272
Spagnolo JF, Hogue BG (2000) Host protein interactions with the 3′ end of bovine coronavirus RNA and the requirement of the poly(A) tail for coronavirus defective genome replication. J Virol 74:5053–5065
Stertz S, Reichelt M, Spiegel M, Kuri T, Martinez-Sobrido L, Garcia-Sastre A, Weber F, Kochs G (2007) The intracellular sites of early replication and budding of SARS-coronavirus. Virology 361:304–315
Supekova L, Supek F, Lee J, Chen S, Gray N, Pezacki JP, Schlapbach A, Schultz PG (2008) Identification of human kinases involved in hepatitis C virus replication by small interference RNA library screening. J Biol Chem 283:29–36
Surjit M, Liu B, Chow VT, Lal SK (2006) The nucleocapsid protein of severe acute respiratory syndrome-coronavirus inhibits the activity of cyclin-cyclin-dependent kinase complex and blocks S phase progression in mammalian cells. J Biol Chem 281:10669–10681
Taguchi F, Siddell SG (1985) Difference in sensitivity to interferon among mouse hepatitis viruses with high and low virulence for mice. Virology 147:41–48
Takano T, Katoh Y, Doki T, Hohdatsu T (2013) Effect of chloroquine on feline infectious peritonitis virus infection in vitro and in vivo. Antiviral Res 99:100–107
Tan YW, Hong W, Liu DX (2012) Binding of the 5′-untranslated region of coronavirus RNA to zinc finger CCHC-type and RNA-binding motif 1 enhances viral replication and transcription. Nucleic Acids Res 40:5065–5077
Tanaka Y, Sato Y, Osawa S, Inoue M, Tanaka S, Sasaki T (2012) Suppression of feline coronavirus replication in vitro by cyclosporin A. Vet Res 43:41
Tanaka Y, Sato Y, Sasaki T (2013) Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 5:1250–1260
Tanaka Y, Sato Y, Sasaki T (2016) Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J Gen Virol 98:190–200
Teigelkamp S, Achsel T, Mundt C, Gothel SF, Cronshagen U, Lane WS, Marahiel M, Luhrmann R (1998) The 20kD protein of human [U4/U6.U5] tri-snRNPs is a novel cyclophilin that forms a complex with the U4/U6-specific 60kD and 90kD proteins. RNA 4:127–141
Thornbrough JM, Jha BK, Yount B, Goldstein SA, Li Y, Elliott R, Sims AC, Baric RS, Silverman RH, Weiss SR (2016) Middle East respiratory syndrome coronavirus NS4b protein inhibits host RNase L activation. MBio 7:e00258
Totura AL, Baric RS (2012) SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol 2:264–275
Totura AL, Whitmore A, Agnihothram S, Schafer A, Katze MG, Heise MT, Baric RS (2015) Toll-Like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio 6:e00638–e00615
Tresnan DB, Levis R, Holmes KV (1996) Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J Virol 70:8669–8674
Ulasli M, Verheije MH, de Haan CA, Reggiori F (2010) Qualitative and quantitative ultrastructural analysis of the membrane rearrangements induced by coronavirus. Cell Microbiol 12:844–861
van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, Osterhaus AD, Haagmans BL, Gorbalenya AE, Snijder EJ, Fouchier RA (2012) Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 3:e00473–e00412
van der Hoek L (2007) Human coronaviruses: what do they cause? Antiviral therapy 12:651–658
van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B (2004) Identification of a new human coronavirus. Nat Med 10:368–373
van der Hoeven B, Oudshoorn D, Koster AJ, Snijder EJ, Kikkert M, Barcena M (2016) Biogenesis and architecture of arterivirus replication organelles. Virus Res 220:70–90
van der Meer Y, Snijder EJ, Dobbe JC, Schleich S, Denison MR, Spaan WJ, Krijnse Locker J (1999) Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J Virol 73:7641–7657
van der Schaar HM, Dorobantu CM, Albulescu L, Strating JR, van Kuppeveld FJ (2016) Fat(al) attraction: picornaviruses usurp lipid transfer at membrane contact sites to create replication organelles. Trends Microbiol 24:535–546
van Hemert MJ, van den Worm SH, Knoops K, Mommaas AM, Gorbalenya AE, Snijder EJ (2008) SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog 4:e1000054
van Kasteren PB, Bailey-Elkin BA, James TW, Ninaber DK, Beugeling C, Khajehpour M, Snijder EJ, Mark BL, Kikkert M (2013) Deubiquitinase function of arterivirus papain-like protease 2 suppresses the innate immune response in infected host cells. Proc Natl Acad Sci USA 110:E838–E847
Verheije MH, Raaben M, Mari M, Te Lintelo EG, Reggiori F, van Kuppeveld FJ, Rottier PJ, de Haan CA (2008) Mouse hepatitis coronavirus RNA replication depends on GBF1-mediated ARF1 activation. PLoS Pathog 4:e1000088
Versteeg GA, Slobodskaya O, Spaan WJ (2006) Transcriptional profiling of acute cytopathic murine hepatitis virus infection in fibroblast-like cells. J Gen Virol 87:1961–1975
Vijay R, Perlman S (2016) Middle East respiratory syndrome and severe acute respiratory syndrome. Curr Opin Virol 16:70–76
Vijgen L, Keyaerts E, Lemey P, Maes P, Van Reeth K, Nauwynck H, Pensaert M, Van Ranst M (2006) Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J Virol 80:7270–7274
Vogels MW, van Balkom BW, Kaloyanova DV, Batenburg JJ, Heck AJ, Helms JB, Rottier PJ, de Haan CA (2011) Identification of host factors involved in coronavirus replication by quantitative proteomics analysis. Proteomics 11:64–80
von Brunn A, Ciesek S, von Brunn B, Carbajo-Lozoya J (2015) Genetic deficiency and polymorphisms of cyclophilin a reveal its essential role for human coronavirus 229E replication. Curr Opin Virol 14:56–61
Walls AC, Tortorici MA, Bosch BJ, Frenz B, Rottier PJ, DiMaio F, Rey FA, Veesler D (2016) Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature 531:114–117
Walsh D, Mohr I (2011) Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol 9:860–875
Wang Y, Liu L (2016) The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a toll-like-receptor-related TRAF3-independent mechanism. MBio 7:e01872–e01815
Wang X, Liao Y, Yap PL, Png KJ, Tam JP, Liu DX (2009) Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J Virol 83:12462–12472
Wang G, Chen G, Zheng D, Cheng G, Tang H (2011) PLP2 of mouse hepatitis virus A59 (MHV-A59) targets TBK1 to negatively regulate cellular type I interferon signaling pathway. PLoS ONE 6:e17192
Wang D, Fang L, Shi Y, Zhang H, Gao L, Peng G, Chen H, Li K, Xiao S (2015) Porcine epidemic diarrhea virus 3C-like protease regulates its interferon antagonism by cleaving NEMO. J Virol 90:2090–2101
Wathelet MG, Orr M, Frieman MB, Baric RS (2007) Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J Virol 81:11620–11633
WHO (2004) SARS website. http://www.who.int/csr/sars/country/table2004_04_21/
WHO (2016) http://www.who.int/emergencies/mers-cov/en/
Wilkins C, Gale M Jr (2010) Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 22:41–47
Williams RK, Jiang GS, Holmes KV (1991) Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A 88:5533–5536
Wong HH, Kumar P, Tay FP, Moreau D, Liu DX, Bard F (2015) Genome-wide screen reveals valosin-containing protein requirement for coronavirus exit from endosomes. J Virol 89:11116–11128
Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY (2005) Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895
Wu K, Li W, Peng G, Li F (2009) Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc Natl Acad Sci U S A 106:19970–19974
Wu CH, Chen PJ, Yeh SH (2014) Nucleocapsid phosphorylation and RNA helicase DDX1 recruitment enables coronavirus transition from discontinuous to continuous transcription. Cell Host Microbe 16:462–472
Xiao H, Xu LH, Yamada Y, Liu DX (2008) Coronavirus spike protein inhibits host cell translation by interaction with eIF3f. PLoS ONE 3:e1494
Xu L, Khadijah S, Fang S, Wang L, Tay FP, Liu DX (2010) The cellular RNA helicase DDX1 interacts with coronavirus nonstructural protein 14 and enhances viral replication. J Virol 84:8571–8583
Xu LH, Huang M, Fang SG, Liu DX (2011) Coronavirus infection induces DNA replication stress partly through interaction of its nonstructural protein 13 with the p125 subunit of DNA polymerase delta. J Biol Chem 286:39546–39559
Yang D, Leibowitz JL (2015) The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res 206:120–133
Yang N, Ma P, Lang J, Zhang Y, Deng J, Ju X, Zhang G, Jiang C (2012) Phosphatidylinositol 4-kinase IIIbeta is required for severe acute respiratory syndrome coronavirus spike-mediated cell entry. J Biol Chem 287:8457–8467
Yang Y, Zhang L, Geng H, Deng Y, Huang B, Guo Y, Zhao Z, Tan W (2013) The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell 4:951–961
Yang Y, Du L, Liu C, Wang L, Ma C, Tang J, Baric RS, Jiang S, Li F (2014) Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 111:12516–12521
Yang Y, Liu C, Du L, Jiang S, Shi Z, Baric RS, Li F (2015) Two mutations were critical for bat-to-human transmission of middle east respiratory syndrome coronavirus. J Virol 89:9119–9123
Ye Y, Hauns K, Langland JO, Jacobs BL, Hogue BG (2007) Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist. J Virol 81:2554–2563
Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, Holmes KV (1992) Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357:420–422
Yu W, Leibowitz JL (1995) Specific binding of host cellular proteins to multiple sites within the 3′ end of mouse hepatitis virus genomic RNA. J Virol 69:2016–2023
Yuan X, Yao Z, Wu J, Zhou Y, Shan Y, Dong B, Zhao Z, Hua P, Chen J, Cong Y (2007) G1 phase cell cycle arrest induced by SARS-CoV 3a protein via the cyclin D3/pRb pathway. Am J Respir Cell Mol Biol 37:9–19
Yuan L, Chen Z, Song S, Wang S, Tian C, Xing G, Chen X, Xiao ZX, He F, Zhang L (2015) p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J Biol Chem 290:3172–3182
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820
Zalinger ZB, Elliott R, Rose KM, Weiss SR (2015) MDA5 is critical to host defense during infection with murine coronavirus. J Virol 89:12330–12340
Zeng Q, Langereis MA, van Vliet AL, Huizinga EG, de Groot RJ (2008) Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci U S A 105:9065–9069
Zhang X, Lai MM (1995) Interactions between the cytoplasmic proteins and the intergenic (promoter) sequence of mouse hepatitis virus RNA: correlation with the amounts of subgenomic mRNA transcribed. J Virol 69:1637–1644
Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, Wu J (2007) Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology 365:324–335
Zhang L, Zhang ZP, Zhang XE, Lin FS, Ge F (2010) Quantitative proteomics analysis reveals BAG3 as a potential target to suppress severe acute respiratory syndrome coronavirus replication. J Virol 84:6050–6059
Zhao Z, Thackray LB, Miller BC, Lynn TM, Becker MM, Ward E, Mizushima NN, Denison MR, Virgin HWt (2007) Coronavirus replication does not require the autophagy gene ATG5. Autophagy 3:581–585
Zhao L, Jha BK, Wu A, Elliott R, Ziebuhr J, Gorbalenya AE, Silverman RH, Weiss SR (2012) Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe 11:607–616
Zheng B, He ML, Wong KL, Lum CT, Poon LL, Peng Y, Guan Y, Lin MC, Kung HF (2004) Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J Interferon Cytokine Res 24:388–390
Zheng D, Chen G, Guo B, Cheng G, Tang H (2008) PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res 18:1105–1113
Zhou P, Li H, Wang H, Wang LF, Shi Z (2012) Bat severe acute respiratory syndrome-like coronavirus ORF3b homologues display different interferon antagonist activities. J Gen Virol 93:275–281
Zust R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS, Siddell SG, Ludewig B, Thiel V (2011) Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol 12:137–143
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Wilde, A.H., Snijder, E.J., Kikkert, M., van Hemert, M.J. (2017). Host Factors in Coronavirus Replication. In: Tripp, R., Tompkins, S. (eds) Roles of Host Gene and Non-coding RNA Expression in Virus Infection. Current Topics in Microbiology and Immunology, vol 419. Springer, Cham. https://doi.org/10.1007/82_2017_25
Download citation
DOI: https://doi.org/10.1007/82_2017_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05368-0
Online ISBN: 978-3-030-05369-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)