Abstract
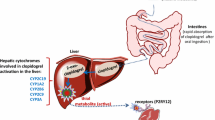
Celecoxib is the only FDA selective cyclooxygenase-2 (COX-2) inhibitor approved nowadays. Studies showed that its therapeutic efficacy and toxicity may be related to inter-individual variability in pharmacodynamics and pharmacokinetics. This review aims to give an updated overview on celecoxib pharmacodynamics and pharmacokinetics in relation to genetics. For this purpose, a Medline search was performed to collect relevant literature between 2004 and 2014. Studies showed that single nucleotide polymorphism (SNP) in PTGS2-765G>C does not control COX-2 inhibitory effect of celecoxib. PGTS1 mutations may have an impact on the selectivity of celecoxib and as such on its gastrointestinal adverse events. Moreover, CYP2C9*2 and *3 allele were identified in bleeding patients taking celecoxib versus control patients. CYP2C9*2, CYP2C*3, two PTGS1 SNPs, and other variant genotypes have shown an association with acute coronary syndromes in patients taking celecoxib. As for the metabolism of celecoxib, in vitro and ex vivo studies showed a reduced clearance of celecoxib in individuals carrier of CYP2C9*3 allele and to a lower extent with CYP2C9*2 carriers. Studies also demonstrated that CYP2C8 does not have a major role in the metabolism of celecoxib. Non-conclusive data are found on the Uridine diphosphate glucuronosyltransferases (UGTs) which catalyze the second phase of the metabolism of celecoxib. As a conclusion, celecoxib should be used with caution in patients known or suspected to be poor CYP2C9 metabolizers. As for CYP2C8, UGTs and other genotypes further studies are still needed to confirm their role in the administration of celecoxib to the right person at the right dose and right time.
Similar content being viewed by others
References
ADAPT Research Group (2006) Cardiovascular and cerebrovascular events in the randomized controlled Alzheimer’s disease anti-inflammatory prevention trial (ADAPT). PLoS Clin Trials 1:e33. doi:10.1371/journal.pctr.0010033
Ali ZK, Kim RJ, Ysla FM (2009) CYP2C9 polymorphisms: considerations in NSAID therapy. Curr Opin Drug Discov Dev 12(1):108–114
Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J et al (2006) Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355:885–895. doi:10.1056/NEJMoa061652
Barkin RL (2012) Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. doi:10.1097/MJT.0b013e3182459abd
Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K et al (2006) Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 355:873–884. doi:10.1056/NEJMoa061355
Bielinski SJ, Olson JE, Pathak J, Weinshilboum RM, Wang L et al (2014) Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc 89(1):25–33. doi:10.1016/j.mayocp.2013.10.021
Brunton LL, Chabner BA, Knollmann BC (eds) (2011). Anti-inflammatory, antipyretic & analgesic agents; pharmacotherapy of gout. In: Goodman & Gilman’s the pharmacological basis of therapeutics, 12th edn, Chapter 34, McGraw-Hill, New York
Chan AT, Zauber AN, Hsu M, Breazna A, Hunter DJ, Rosenstein RB, Eagle CJ, Hawk ET, Bertagnolli MM (2009) Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gatroenterology 136:2127–2136. doi:10.1053/j.gastro.2009.02.045
Cipollone F, Toniato E, Martinotti S, Fazia M, Iezzi A, Cuccurullo C et al (2004) A polymorphism in the cyclooxygenase 2 gene as an inherited protective factor against myocardial infarction and stroke. J Am Med Assoc 291:2221–2228. doi:10.1001/jama.291.18.2221
Daily EB, Aquilante CL (2009) Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies. Pharmacogenomics 10(9):1489–1510. doi:10.2217/pgs.09.82
Dogne JM, De Leval X, Hanson J, Frederich M, Lambermont B, Ghuysen A et al (2004) New developments on thromboxane and prostacyclin modulators. Part I: thromboxane modulators. Curr Med Chem 11:1223–1241. doi:10.2174/0929867043365260
FDA (2011). Celebrex® (Celecoxib) capsules. http://www.fda.gov
Fries S, Grosser T, Price TS, Lawson JA, Kapoor S, DeMarco S, Pletcher MT, Wiltshire T, FitzGerald GA (2006) Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology 130(1):55–64. doi:10.1053/j.gastro.2005.10.002
Gharani N, Keller MA, Stack CB, Hodges LM, Schmidlen TJ et al (2013) The Coriell personalized medicine collaborative pharmacogenetics appraisal, evidence scoring and interpretation system. Genome Med 5:93. doi:10.1186/gm499
Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RN, Klein TE (2012) Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics 22(4):310–318. doi:10.1097/FPC.0b013e32834f94cb
Harper K, Tyson-Capper AJ (2008) Complexity of COX-2 gene regulation. Biochem Soc Trans 36(Pt 3):543–545. doi:10.1042/BST0360543
Ishizaki T (2002) CYP2C pharmacogenetics and tailor-made therapeutic implications. Int Congr Ser 1244:1–9
Kapur BM, Lala PK, Shaw JLV (2014) Pharmacogenetics of chronic pain management. Clin Biochem 47:1169–1187. doi:10.1016/j.clinbiochem.2014.05.065
Karjalainen MJ, Neuvonen PJ, Backman JT (2008) In vitro inhibition of CYP1A2 by model inhibitors, anti-inflammatory analgesics and female sex steroids: predictability of in vivo interactions. Basic Clin Pharmacol Toxicol 103:157–165. doi:10.1111/j.1742-7843.2008.00252.x
Kraus S, Hummler S, Toriola AT, Poole EM, Scherer D, Kotzmann J et al (2013) Impact of genetic polymorphisms on adenoma recurrence and toxicity in a COX2 inhibitor (celecoxib) trial: results from a pilot study. Pharmacogenet Genomics 23(8):428–437. doi:10.1097/FPC.0b013e3283631784
Pilotto A, Seripa D, Franceschi M, Scarcelli C, Colaizzo D, Grandone E, Nitro V, Andriulli A, Leandro G, Di Mario F, Dallapiccola B (2007) Genetic susceptibility to nonsteroidal anti-inflammatory drug-related gastroduodenal bleeding: role of cytochrome P450 2C9 polymorphisms. Gatroenterology 133(2):465–471. doi:10.1053/j.gastro.2007.05.025
Praveen Rao PN, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11(2):81s–110s
Prieto-Perez R, Ochoa D, Cabaleiro T, Roman M, Sanchez-Rojas SD et al (2013) Evaluation of the relationship between polymophisms in CYP2C8 and CYP2C9 and the pharmacokinetics of celecoxib. J Clin Pharmacol 53(12):1261–1267. doi:10.1002/jcph.169
Rollason V, Samer CF, Daali Y, Desmeules JA (2014) Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti-inflammatory drugs: a review. Curr Drug Metab 15:326–343. doi:10.2174/1389200215666140202214454
Shi S, Klotz U (2008) Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol 64:233–252. doi:10.1007/s00228-007-0400-7
Shi W, Wang YM, Li D, Chen NN, Chen BY (2004) Safety and efficacy of oral non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a six-month randomized study. Clin Drug Investig 24:89–101
Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS et al (2006) Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med 119:255–266. doi:10.1016/j.amjmed.2005.09.054
Skarte C, Reus M, Schmidt R, Grundei I, Schuss P et al (2006) The cyclooxygenase 2 genetic variant-765G>C does not modulate the effects of celecoxib on prostaglandin E2 production. Clin Pharmacol Ther 80(6):621–632. doi:10.1016/j.clpt.2006.05.021
St. Germaine CG, Bogaty P, Boyer L, Hanley J, Engert JC, Brophy JM (2010) Genetic polymorphisms and the cardiovascular risk of non-steroidal anti-inflammatory drugs. Am J Cardiol 105:1740–1745. doi:10.1016/j.amjcard.2010.01.352
Wyatt JE, Pettit WL, Harirforoosh S (2012) Pharmacogenetics of nonsteroidal anti-inflammatory drugs. Pharmacogenomics J 12:462–467. doi:10.1038/tpj.2012.40
Author information
Authors and Affiliations
Corresponding author
Additional information
Endorsed by Asser Ghoneim.
Rights and permissions
About this article
Cite this article
Domiati, S., Ghoneim, A. Celecoxib for the Right Person at the Right Dose and Right Time: An Updated Overview. Springer Science Reviews 3, 137–140 (2015). https://doi.org/10.1007/s40362-015-0034-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40362-015-0034-6