Abstract
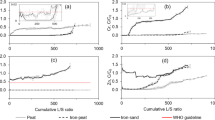
Colloidal pyrite waste rocks (CPWR) which are mainly composed of colloidal pyrite and siderite widely deposit in sulfide mines in the middle and lower reaches of the Yangtze River belt, China, especially in Tongling City, Anhui Province, China. In this paper, a fixed-bed column was used to investigate the weathering and oxidation of CPWR and its role in immobilizing low-concentration Cu (10 mg L−1) under weakly acidic leach (pH = 5.0). The experimental results indicated that the breakthrough capacity was around 14.0 mg Cu g−1 CPWR when Cu2+ breakthrough concentration was 0.5 mg L−1. Sequential extraction of Cu and dithionite–citrate–bicarbonate extraction of free iron in the used CPWR after the column breakthrough indicated that Cu removal by CPWR consisted of the following processes: oxidation of pyrite and dissolution of siderite in CPWR, ferrous oxidation, and adsorption of Cu on ferric (hydr)oxides. This study shows the potential application of CPWR as an effective sorbent for the removal of low-concentration Cu from acid mine drainage.






Similar content being viewed by others
References
Bénézeth P, Dandurand JL, Harrichoury JC (2009) Solubility product of siderite (FeCO3) as a function of temperature (25–250 °C). Chem Geol 265:3–12. doi:10.1016/j.chemgeo.2009.03.015
Bower J, Savage KS, Weinman B, Barnett MO, Hamilton WP, Harper WF (2008) Immobilization of mercury by pyrite (FeS2). Environ Pollut 156:504–514. doi:10.1016/j.envpol.2008.01.011
Caldeira CL, Ciminelli VST, Osseo-Asare K (2010) The role of carbonate ions in pyrite oxidation in aqueous systems. Geochim Cosmochim Acta 74:1777–1789. doi:10.1016/j.gca.2009.12.014
Cerón JC, Grande JA, Torre ML, Santisteban M, Valente T (2013) Impact of AMD processes on the water dams of the Iberian Pyrite Belt: overall hydrochemical characterization (Huelva, SW Spain). Water Air Soil Pollut 224:1642–1652. doi:10.1007/s11270-013-1642-x
Chandra AP, Gerson AR (2010) The mechanisms of pyrite oxidation and leaching: a fundamental perspective. Surf Sci Rep 65:293–315. doi:10.1016/j.surfrep.2010.08.003
Chandra AP, Puskar L, Simpson DJ, Gerson AR (2012) Copper and xanthate adsorption onto pyrite surfaces: implications for mineral separation through flotation. Int J Miner Process 114–117:16–26. doi:10.1016/j.minpro.2012.08.003
Chen TH, Yang Y, Chen D, Li P, Shi YD, Zhu X (2013) The structural evolution of heat-treated colloidal pyrite under inert atmosphere and its application for the purification of Cu(II) ion from wastewater. Environ Eng Manag J 12:1411–1416
Demoisson F, Mullet M, Humbert B (2007) Investigation of pyrite oxidation by hexavalent chromium: solution species and surface chemistry. J Colloid Interface Sci 316:531–540. doi:10.1016/j.jcis.2007.08.011
Donato PD, Mustin C, Benoit R, Erre R (1993) Spatial distribution of iron and sulphur species on the surface of pyrite. Appl Surf Sci 68:81–93. doi:10.1016/0169-4332(93)90217-Y
Equeenuddin SM (2014) Occurrence of alpersite at Malanjkhand copper mine, India. Environ Earth Sci 73:3849–3853. doi:10.1007/s12665-014-3668-9
Futalan CM, Kan C-C, Dalida ML, Pascua C, Wan M-W (2011) Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr Polym 83:697–704. doi:10.1016/j.carbpol.2010.08.043
Grande JA, Santisteban M, de la Torre ML, Valente T, Perez-Ostale E (2013) Characterisation of AMD pollution in the reservoirs of the Iberian Pyrite Belt. Mine Water Environ 32:321–330. doi:10.1007/s10230-013-0236-6
Guo H, Ren Y, Liu Q, Zhao K, Li Y (2013) Enhancement of arsenic adsorption during mineral transformation from siderite to goethite: mechanism and application. Environ Sci Technol 47:1009–1016. doi:10.1021/es303503m
Han R, Zou W, Li H, Li Y, Shi J (2006) Copper(II) and lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J Hazard Mater 137:934–942. doi:10.1016/j.jhazmat.2006.03.016
Integrated Wastewater Discharge Standard (1996) State Bureau of Technology Supervision. http://kjs.mep.gov.cn/hjbhbz/bzwb/shjbh/swrwpfbz/199801/W020061027521858212955.pdf
Jeong HY, Han Y-S, Park SW, Hayes KF (2010a) Aerobic oxidation of mackinawite (FeS) and its environmental implication for arsenic mobilization. Geochim Cosmochim Acta 74:3182–3198. doi:10.1016/j.gca.2010.03.012
Jeong HY, Sun K, Hayes KF (2010b) Microscopic and spectroscopic characterization of Hg(II) immobilization by mackinawite (FeS). Environ Sci Technol 44:7476–7483
Kelly WC, Turneaure FS (1970) Mineralogy, paragenesis and geothermometry of the tin and tungsten deposits of the eastern Andes. Bolivia Econ Geol 65:609–680
Lin Y-T, Huang C-P (2008) Reduction of chromium(VI) by pyrite in dilute aqueous solutions. Sep Purif Technol 63:191–199. doi:10.1016/j.seppur.2008.05.001
Malakooti SJ, Tonkaboni SZS, Noaparast M, Ardejani FD, Naseh R (2013) Characterisation of the Sarcheshmeh copper mine tailings, Kerman province, southeast of Iran. Environ Earth Sci 71:2267–2291. doi:10.1007/s12665-013-2630-6
Martín-Crespo T, De Ignacio-San José C, Gómez-Ortiz D, Martín-Velázquez S, Lillo-Ramos J (2009) Monitoring study of the mine pond reclamation of Mina Concepción, Iberian Pyrite Belt (Spain). Environ Earth Sci 59:1275–1284. doi:10.1007/s12665-009-0115-4
Martínez CE, McBride MB (1998) Solubility of Cd2+, Cu2+, Pb2+, and Zn2+ in aged coprecipitates with amorphous iron hydroxides. Environ Sci Technol 32:743–748. doi:10.1021/es970262+
Mehra OP, Jackson ML (1960) Iron oxides removed from soils and clays by dithionote–citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:317–327
Moses CO, Herman JS (1991) Pyrite oxidation at circumneutral pH. Geochim Cosmochim Acta 55:471–482. doi:10.1016/0016-7037(91)90005-P
Moses CO, KirkNordstrom D, Herman JS, Mills AL (1987) Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochimica et Cosmochimica Acta 51:1561–1571
Raven KP, Jain A, Loeppert RH (1998) Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ Sci Technol 32:344–349
Rimstidt JD, Vaughan DJ (2003) Pyrite oxidation: a state-of-the-art assessment of the reaction mechanism. Geochim Cosmochim Acta 67:873–880. doi:10.1016/s0016-7037(02)01165-1
Romero FM, Nunez L, Gutierrez ME, Armienta MA, Ceniceros-Gomez AE (2011) Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area. Mexico Arch Environ Contam Toxicol 60:191–203. doi:10.1007/s00244-010-9544-z
Sahinkaya E, Gunes FM, Ucar D, Kaksonen AH (2011) Sulfidogenic fluidized bed treatment of real acid mine drainage water. Bioresour Technol 102:683–689. doi:10.1016/j.biortech.2010.08.042
Sahoo PK, Tripathy S, Panigrahi MK, Equeenuddin SM (2013) Inhibition of acid mine drainage from a pyrite-rich mining waste using industrial by-products: role of neo-formed phases. Water Air Soil Pollut 224:1757–1767. doi:10.1007/s11270-013-1757-0
Sainz A, Ruiz F (2006) Influence of the very polluted inputs of the Tinto-Odiel system on the adjacent littoral sediments of southwestern spain: a statistical approach. Chemosphere 62:1612–1622. doi:10.1016/j.chemosphere.2005.06.045
Shokri BJ, Ramazi H, Ardejani FD, Moradzadeh A (2013) A statistical model to relate pyrite oxidation and oxygen transport within a coal waste pile: case study, Alborz Sharghi, northeast of Iran. Environ Earth Sci 71:4693–4702. doi:10.1007/s12665-013-2859-0
Stookey LL (1970) Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Todd EC, Sherman DM, Purton JA (2003) Surface oxidation of pyrite under ambient atmospheric and aqueous (pH = 2 to 10) conditions: electronic structure and mineralogy from X-ray absorption spectroscopy. Geochim Cosmochim Acta 67:881–893. doi:10.1016/S0016-7037(02)00957-2
Wilkie JA, Hering JG (1996) Adsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surf A 107:97–110. doi:10.1016/0927-7757(95)03368-8
World Health Organization (2005) Nickel in drinking water: background document for development of WHO. World Health Organization. http://www.who.int/water_sanitation_health/gdwqrevision/nickel2005.pdf
Xu XC, Xie Q, Chen F, Wang J, Wu W (2008) Acid mine drainage and heavy metal pollution from solid waste in the Tongling Mines. China Acta Geologia Sinica 82:146–153
Yang Y, Chen T, Li P, Liu H, Xie J, Xie Q, Zhan X (2014) Removal and recovery of Cu and Pb from single-metal and Cu–Pb–Cd–Zn multimetal solutions by modified pyrite: fixed-bed columns. Ind Eng Chem Res 53:18180–18188. doi:10.1021/ie503828f
Yousefi S, Ardejani FD, Ziaii M, Abedi A, Zadeh EE (2014) Investigating the origin and geochemical behaviour of toxic elements within the waste dumps using statistical analyses: a case study at waste dumps of Sarcheshmeh copper mine, SE of Iran. Environ Earth Sci 73:1555–1572. doi:10.1007/s12665-014-3507-z
Zhang M (2011) Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem Eng J 172:361–368. doi:10.1016/j.cej.2011.06.017
Zhou T, Yuan F, Yue S, Zhao Y (2000) Two series of copper-gold deposits in the middle and lower reaches of the Yangtze River area (MLYRA) and the hydrogen, oxygen, sulfur and lead isotopes of their ore-forming hydrothermal systems. Sci China Ser D Earth Sci 43:208–218. doi:10.1007/bf02911946
Acknowledgments
This study was financially supported by the Natural Science Foundation of China (No. 41130206 and 41402029). Yan Yang is grateful for the funding provided by the China Scholarship Council (No. 201306690001) and European Cooperation in Science and Technology (COST Action Programs 1205 and 1302).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Chen, T., Li, P. et al. Immobilization of copper under an acid leach of colloidal pyrite waste rocks by a fixed-bed column. Environ Earth Sci 75, 205 (2016). https://doi.org/10.1007/s12665-015-4991-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4991-5