Abstract
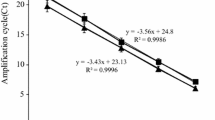
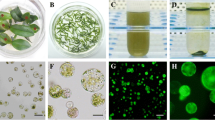
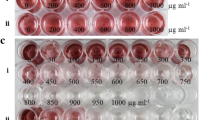
The regeneration of Candida glycerinogenes protoplasts is a major step following genetic manipulations such as fusion and DNA-mediated transformation. An investigation of protoplast formation and cytological examination was used to gain further insight into the loss of protoplast viability in osmotically stabilized support media. Protoplasts with the highest regeneration frequency (98.6% protoplasts/mL) were isolated, using lysozyme dissolved in 1M sorbitol osmoticum. The commercial enzyme preparations, osmotic stabilisers, and growth phase were effective in raising the protoplast yield. Sodium chloride was effective for protoplast preparation; however, sugars and sugar alcohols were better for protoplast regeneration. Sorbitol at a concentration of 1 M was used in regeneration agar for further studies. Regeneration of colonies from protoplasts was maximal (11 ~ 15%) when protoplasts were incorporated in cooled agar containing 0.5% glucose, supplemented with 1M sorbitol as osmotic stabilizer. C. glycerinogenes strain was highly sensitive to zeocin, so transformation of protoplasts and PEG-mediated was achieved with an improved transformation system, using plasmid pURGAP-gfp containing zeocin gene driven by a PCgGAP promoter from C. glycerinogenes to express gfp gene and be transformed into the 5.8S rDNA site of C. glycerinogenes in order to test the system for studying the yeast osmoregulation. We developed an efficient method for transformation of C. glycerinogenes, and parameters involved in transformation efficiency were optimized. Expressions of gfp at different levels were conducted under osmotic stress containing NaCl, KCl, sorbitol or glycerol for the recombinant strains. These improved procedures for protoplast isolation, regeneration and transformation proved to be useful applications in genetic studies for other Candida species and industrial yeast.
Similar content being viewed by others
References
Murlidhar, R. V. and T. Panda (2000) Fungal protoplast fusion: A revisit. Bioproc. Eng. 22: 429–431.
Hopwood, D. A. (1981) Genetic studies with bacterial protoplasts. Annu. Rev. Microbiol. 35: 237–272.
Illingg, T. (1987) Protoplast fusion and regeneration in Streptomyces clavuligerus. Ph. D. Thesis. University of Nottingham, Nottingham.
Zhuge, J., H. Y. Fang, Z. X. Wang, D. Z. Chen, H. R. Jin, and H. L. Gu (2001) Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl. Microbiol. Biotechnol. 55: 686–692.
Chen, X. Z., H. Y. Fang, Z. M. Rao, W. Shen, B. Zhuge, Z. X. Wang, and J. Zhuge (2008) Cloning and characterization of a NAD+-dependent glycerol-3-phosphate dehydrogenase gene from Candida glycerinogenes, an industrial glycerol producer. FEMS Yeast Res. 8: 725–734.
Wang, Z. X., J. Zhuge, H. Y. Fang, and B. A. Prior (2001) Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 19: 201–223.
Jin, H. R., H. Fang, and J. Zhuge (2003) By-product formation by a novel glycerol-producing yeast, Candida glycerinogenes, with different O2 supplies. Biotechnol. Lett. 25: 311–314.
Kohler, G. A., T. C. White, and N. Agabian (1997) Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179: 2331–2338.
Walther, A. and J. Wendland (2003) An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42: 339–343.
Fincham, J. R. S. (1989) Transformation in fungi. Microbiol. Rev. 53: 148–170.
Zhang, C., H. Zong, B. Zhuge, X. Y. Lu, H. Y. Fang, and J. Zhuge (2015) Integrative expression vectors for overexpression of xylitol dehydrogenase (XYL2) in osmotolerant yeast, Candida glycerinogenes WL2002–5. J. Ind. Microbiol. Biot. 42: 113–124.
Zhang, C., B. Zhuge, X. B. Zhan, H. Y. Fang, H. Zong, and J. Zhuge (2013) Cloning and characterization of a novel NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase gene from Candida glycerinogenes and use of its promoter. Yeast. 30: 157–163.
Moriguchi, M. and S. Kotagawa (1994) Preparation and regeneration of Aspergellus awamori. Lett. Appl. Microbiol. 18: 30–31.
Chen, X. Z., H. Y. Fang, Z. M. Rao, W. Shen, B. Zhuge, Z. X. Wang, and J. Zhuge (2008) An efficient genetic transformation method for glycerol producer Candida glycerinogenes. Microbiol. Res. 163: 531–537.
Sambrook, J. and D. W. Russell (2001) Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA.
Mann, W. and J. Jeffery (1986) Yeast in molecular biology, spheroplast preparation with Candida utilis, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Biosci. Rep. 6: 597–602.
Kitamoto, Y., N. Mo, T. Ohiwa, and Y. Ichikawa (1988) A simple method for protoplast formation and improvement of protoplast regeneration from various fungi using an enzyme from Trichoderma harzianum. Appl. Microbiol. Biotechnol. 28: 445–450.
Kirn, B. K., J. H. Kang, M. Jin, H. W. Kim, M. J. Shim, and E. C. Choi (2000) Mycelial protoplast isolation and regeneration in Lentinus lepideus. Life. Sci. 66: 1359–1367.
Gupta, U., G. S. Cheema, H. S. Sodhi, and R. P. Phutela (1997) Protoplast isolation and regeneration in Agaricus bisporus strain MS 39. Mush. Res. 6: 59–62.
Vijaya, P. P. (1995) Biochemical, physiological and molecular aspects of penconazole and carbendazim resistance in mutants and protoplast fusants of Venturia inaequalis (Cooke) Wint. Ph. D. Thesis. University of Madras, Chennai, India.
Peberdy, J. F., C. E. Buckley, D. C. Daltrey, and P. M. Moore (1976) Factors affecting protoplast release in some flamentous fungi. Transactions of the British Mycol. Soc. 67: 23–26.
Curragh, H. J., H. Mooibroek, J. G. H. Wessels, R. Marchant, and E. Mullan (1992) Protoplast formation and DNA-mediated transformation of Fusarium culmorum to hygromycin B resistance. Mycol. Res. 97: 313–317.
Gomez, M. J., K. Luyten, and J. Ramos (1996) The capacity to transport potassium influences sodium tolerance in Saccharomyces cerevisiae. FEMS Microbiol. let. 135: 157–160.
Ansell, R., K. Granath, S. Hohmann, J. M. Thevelein, and L. Adler (1997) The two isoenzymes for yeast NAD+-dependent glycerol-3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. Embo. J. 16: 2179–2187.
Rao, Z. M., Z. Ma, W. Shen, H. Y. Fang, and J. Zhuge (2008) Transformation of industrialized strain Candida glycerinogenes with resistant gene zeocin via Agrobacterium tumefaciens. Curr. Microbiol. 57: 12–17.
Helen, L. R. and W. D. James (2001) Protoplast preparation and transient transformation of Rhizoctonia solani. Mycol. Res. 105: 1295–1303.
Peberdy, J. F. (1979) Fungal protoplasts: Isolation, reversion and fusion. Annu. Rev. Microbiol. 33: 21–39.
Hocart, M. J. and J. F. Peberdy (1989) Protoplast technology and strain selection. pp. 235–257. In Biotechnology of Fungi for Improving Plant Growth. Cambridge University Press, Cambridge, London, UK
Illing, G. T., I. D. Normansell, and J. F. Peberdy (1989) Protoplast isolation and Regeneration in Streptomyces clavuligerus. J. Gen. Microbiol. 135: 2289–2291.
Svoboda, A. (1966) Regeneration of yeast protoplasts in agar gels. Exp. Cell. Res. 10: 640–642.
Sheen, J. (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant. Physiol. 127: 1466–1475.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Zong, H., Zhuge, B. et al. Protoplast preparation and polyethylene glycol (PEG)-mediated transformation of Candida glycerinogenes . Biotechnol Bioproc E 21, 95–102 (2016). https://doi.org/10.1007/s12257-015-0686-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0686-8