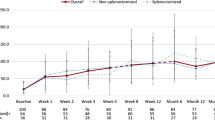
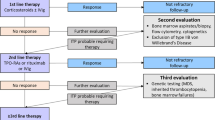
Abstract
Primary immune thrombocytopenia (ITP) is an autoimmune disease mediated by the production of auto-antibody against platelets. Rituximab, an anti-CD20 antibody, is reported to be useful for treatment of ITP. In Japan, however, robust evidence on this treatment has not been accumulated. Hence, we conducted this open-label phase III clinical trial to confirm the efficacy and safety of rituximab, administered at 375 mg/m2 once per week at weekly intervals for 4 consecutive weeks in Japanese patients with chronic ITP, who had relapsed and were refractory to conventional therapy. The primary endpoint was defined as the percentage of patients with a platelet count above 50 × 109/L at week 24 after the first dose of rituximab, which was 30.8 % of 26 patients (95 % confidence interval 14.3–51.8 %). Although the lower confidence limit of primary endpoint failed to meet the pre-specified threshold of 20 %, the clinical efficacy of rituximab is substantial in consideration of the 2 % response rate in the placebo arm in other clinical studies in patients with chronic ITP. We conclude that rituximab is clinically useful and safe in the treatment of Japanese patients with chronic ITP, achieving the goal of maintaining platelet count and reducing risk of bleeding while minimizing treatment-related toxicity.


Similar content being viewed by others
References
Kurata Y, Fujimura K, Kuwana M, Tomiyama Y, Murata M. Epidemiology of primary immune thrombocytopenia in children and adults in Japan: a population-based study and literature review. Int J Hematol. 2011;93:329–35.
Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549–54.
Fujimura K, Kuwana M, Kurata Y, Imamura M, Harada H, Ikeda Y, et al. Is eradication therapy useful as the first line of treatment in Helicobacter pylori-positive idiopathic thrombocytopenic purpura? Analysis of 207 eradicate chronic ITP cases in Japan. Int J Hematol. 2005;81:162–8.
Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–34.
Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA, American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207.
Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98:24–33.
Looney RJ. B-cell targeted therapy in diseases other than rheumatoid arthritis. J Rheumatol. 2005;32(Suppl 73):25–8.
Reddy V, Jayne D, Close D, Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res Ther. 2013;15(Suppl 1):S2.
British Committee for Standards in Haematology General Haematology. Task force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120:574–96.
Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–86.
Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33.
Godeau B, Porcher R, Fain O, Lefrère F, Fenaux P, Cheze S, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112:999–1004.
Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403.
Cooper N, Stasi R, Cunningham-Rundles S, Feuerstein MA, Leonard JP, Amadori S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125:232–9.
Zaja F, Vianelli N, Battista M, Sperotto A, Patriarca F, Tomadini V, et al. Earlier administration of rituximab allows higher rate of long-lasting response in adult patients with autoimmune thrombocytopenia. Exp Hematol. 2006;34:571–2.
Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary ITP. Semin Hematol. 2013;50:S18–21.
Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard JF, Cheze S, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124:3228–36.
Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115:2755–62.
Li Z, Mou W, Lu G, Cao J, He X, Pan X, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93:91–8.
Arnold DM, Heddle NM, Carruthers J, Cook DJ, Crowther MA, Meyer RM, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119:1356–62.
Gudbrandsdottir S, Birgens HS, Frederiksen H, Jensen BA, Jensen MK, Kjeldsen L, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121:1976–81.
Tran H, Brighton T, Grigg A, McRae S, Dixon J, Thurley D, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol. 2014;167:243–51.
Ghanima W, Khelif A, Waage A, Michel M, Tjønnfjord GE, Romdhan NB, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:1653–61.
Acknowledgments
This study is a research project of the Ministry of Health, Labour, and Welfare (MHLW) Scientific Research Project. The authors are gratefully indebted to Zenyaku Kogyo Co., Ltd. for the supply of the investigational product and support for preparation of the manuscript, and to Quintiles Transnational Japan Inc. for support in conducting the clinical trial and in preparation of the manuscript, and Center for Clinical Trials, Japan Medical Association (JMACCT) for financial support. We also appreciated CTD Inc. for providing professional advises to our study group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Miyakawa reports non-financial support from Zenyaku Kogyo, grants from Japan Medical Association Center for Clinical Trials (JMACCT), during the conduct of the study; grants and personal fees from Alexion pharmaceutical, personal fees from Daiichi Sankyo, personal fees from Fuji film, personal fees from GlaxoSmithKline, personal fees from Kyowa-Hakko Kirin, personal fees from Ono pharmaceuticals, outside the submitted work; Dr. Kanakura reports grants from Chugai Pharmaceutical Co. Ltd., personal fees from Zenyaku-Kogyo, during the conduct of the study; grants and personal fees from Kyowa-Hakkyo Kirin Co. Ltd., grants and personal fees from Otsuka Pharmaceutical Co. Ltd., grants from Takeda Pharmaceutical Co. Ltd., grants from Teijin Pharma, grants from Janssen Pharmaceutical Co. Ltd., grants and personal fees from Alexion Pharmaceutical Co. Ltd., personal fees from Shire Co. Ltd., grants from Bristol-Myers Squibb Co. Ltd., outside the submitted work; Dr. Yano has nothing to disclose. Dr. Shirasugi reports personal fees from Kyowa-Hakko Kirin, personal fees from Pfizer, personal fees from Ono pharmaceutical, personal fees from Bristol-Myers Squibb, personal fees from Celgene, personal fees from GlaxoSmithKline, outside the submitted work; Dr. Higashihara reports grants and personal fees from Chugai pharmaceutical, during the conduct of the study; grants and personal fees from Kyowa-Hakko Kirin, grants from MSD, grants from Jannsen pharmaceutical, grants from Takeda, personal fees from Sumitomo Dainippon pharma, grants from Teijin, grants from Astellas pharma, personal fees from GlaxoSmithKline, outside the submitted work; Dr. Nishiwaki reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., outside the submitted work; Dr. Okamoto reports grants from Chugai pharmaceuticals, during the conduct of the study; personal fees from KyowaHakkoKirin, personal fees from Takeda, personal fees from Bristol-Myers Squibb, personal fees from Janssen pharmaceutical, personal fees from Celgene, personal fees from Alexion, personal fees from Eisai, grants from GlaxoSmithKline, outside the submitted work; Dr. Katsutani has nothing to disclose. Dr. Nomura has nothing to disclose. Dr. Kikuchi has nothing to disclose. Dr. Abe has nothing to disclose. Dr. Nishikawa has nothing to disclose. Dr. Ozaki reports grants from Japan Medical Association Center for Clinical Trials, during the conduct of the study; personal fees from Nippon Shinyaku, personal fees from Janssen pharmaceutical companies, personal fees from Chugai pharmaceutical, personal fees from Celgene, personal fees from Kyowa-Hakko Kirin pharmaceutical, outside the submitted work; Dr. Ikeda reports personal fees from Chugai pharmaceuticals, during the conduct of the study; personal fees from Novartis, personal fees from Sanofi, personal fees from Daichi Sankyo, outside the submitted work; Dr. Tomiyama reports grants from JMACCT, during the conduct of the study; personal fees from GSK, personal fees from Novartis, personal fees from Kyowa-Hokko Kirin, personal fees from Eisai, personal fees from Sysmex, outside the submitted work; Dr. Fujimura reports non-financial support from Kyowa-Hakko Kirin, during the conduct of the study; non-financial support from Medical support, outside the submitted work.
About this article
Cite this article
Miyakawa, Y., Katsutani, S., Yano, T. et al. Efficacy and safety of rituximab in Japanese patients with relapsed chronic immune thrombocytopenia refractory to conventional therapy. Int J Hematol 102, 654–661 (2015). https://doi.org/10.1007/s12185-015-1887-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1887-9