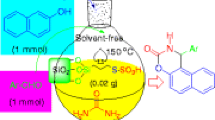
Abstract
A number of methods have been proposed for the modification of the Ritter reaction. However, many of these methods involve the use of strongly acidic conditions, stoichiometric amounts of reagents, harsh reaction conditions and extended reaction times. Therefore, the development of mild, efficient, convenient and benign reagents for the Ritter reaction is desirable. In this research, we have developed a clean and environmentally friendly protocol for the synthesis of amides by using different benzylic or tertiary alcohols and different nitriles in the presence of silica-bonded N- propyl sulphamic acid (SBNPSA) as catalyst under solvent-free conditions in high yields.

A clean and environmentally friendly protocol was developed for the synthesis of amides by using different benzylic or tertiary alcohols and different nitriles in the presence of silica-bonded N- propyl sulphamic acid (SBNPSA) as catalyst under solvent-free conditions in high yields.



Similar content being viewed by others
References
Top S and Jaouen G 1981 J. Org. Chem. 46 78
Justribo V, María I and Colombo M I 2003 Tetrahedron Lett. 44 8023
Clayden J, Greeves N, Warren S and Wothers P 2001 Organic chemistry, New York: Oxford Press
Kurti L and Czako B 2005 Strategic applications of named reactions in organic synthesis, 1st Ed, Elsevier: Amstersdam
Vardanyan R and Hruby V J 2006 Synthesis of essential drugs, 1st Ed. Amsterdam: Elsevier 137
Fujisawa and Deguchi 1958 Chem. Abst. 52 11965
Riego J M, Sedin Z, Zaldivar J M, Marziano N C and Tortato C 1996 Tetrahedron Lett. 37 513. doi:10.1016/0040-4039(95)02174-4
Firouzabadi F and Jafari A A 2005 J. Iran Chem. Soc. 2 85
Salehi P, Zolfigol M A, Shirini F and Baghbanzadeh M 2006 Curr. Org. Chem. 10 2171. doi:10.2174/138527206778742650
Zolfigol M A, Shirini P, Salehi P and Abedini M 2008 Curr. Org. Chem. 12 183. doi:10.2174/138527208783497475
Karimi B and Khalkhali M 2005 J. Mol. Catal. A: Chem. 232 113. doi:10.1016/j.molcata.2005.01.028
Karimi B and Enders D 2006 Org. Lett. 8 1237. doi:10.1021/ol060129z. PMID: 16524312
Zareyee D and Karimi B 2007 Tetrahedron Lett. 48 1277. doi:10.1016/j.tetlet.2006.12.030
Karimi, B and Kalkhali, M 2007 J. Mol. Catal. A: Chem. 271 75. doi:10.1016/j.molcata.2007.02.018
Coma A and Garcia H 2002 Chem. Rev. 102 3837. doi:10.1021/cr010333u
Wight A P and Davis M E 2002 Chem. Rev. 102 3589. doi:10.1021/cr010334m. PMID:12371895
Melero J A, van Grieken R and Morales G 2006 Chem. Rev. 106 3790. doi:10.1021/cr050994h. PMID:16967921
Shimizu K, Hayashi E, Hatamachi T, Kodama T, Higuchi T, Satsuma A and Kitayama Y 2005 J. Catal. 231 131. doi:10.1016/j.jcat.2005.01.017
Niknam K, Zolfigol M A, Khorramabadi-Zad A, Zare R and Shayegh M 2006 Catal. Commun. 7 494. doi: 10.1016/j.catcom.2006.01.004
Niknam K, Karami B and Zolfigol M A 2007 Catal. Commun. 8 1427. doi:10.1016/j.catcom.2006.12.011
Niknam K, Saberi D and Sefat M N 2009 Tetrahedron Lett. 50 4058. doi:10.1016/j.tetlet.2009.04.096
Niknam K, Saberi D and Mohagheghnejad M 2009 Molecules 14 1915. doi:10.3390/molecules14051915. PMID:19471211
Niknam K, Zolfigol M and Sadabadi T 2007 J. Iran Chem. Soc. 4 199
Niknam K and Fatehi-Raviz A 2007 J. Iran Chem. Soc. 4 438
Niknam K, Zolfigol M A, Hossieninejad Z and Daneshvar N 2007 Chin. J. Cata. 28 591. doi:10.1016/S1872-2067(07)60051-5
Niknam K, Zolfigol M A, Sadabadi T and Nejadi A 2006 J. Iran Chem. Soc. 3 318
Niknam K, Zolfigol M A, Razavian S M and Mohammadpoor-Baltork I 2005 Heterocycles 65 657. doi:10.3987/COM-04-10302
Zolfigol M A, Bagherzadeh M, Niknam K, Shirini F, Mohammadpoor-Baltork I, Ghoghamarani A G and Baghbanzadeh M 2006 J. Iran Chem. Soc. 3 73
Niknam K and Zolfigol M A 2006 J. Iran Chem. Soc. 3 59
Niknam K and Daneshvar N 2007 Heterocycles 71 373. doi:10.3987/COM-06-10905
Karimi B and Zareyee D 2005 Tetrahedron Lett. 46 4661
Davies I W, Senanayake C H, Larsen R D, Verhoeven T R and Reider P J 1996 Tetrahedron Lett. 37 813
Thibault-Starzyk F, Payen, R and Lavalley J C 1996 Chem. Commun. 23 2667
Okuhara T, Chen, X and Matsuda H 2001 Appl. Catal. 200 109
Bhushan M K and Virendra R M 2002 Synth. Commun. 32 1731
Concellon J M, Riego E, Suarez J R, Garcia-Granda S and Diaz M R 2004 Org. Lett. 24 4499
Shaoguang S, Zhang Q, Liu Q, Kang J, Yin Y, Li D and Dong D 2005 Tetrahedron Lett. 46 6271
Callens E, Burton A J and Barrett A G M 2006 Tetrahedron Lett. 47 8699
Niknam K, Zolfigol M A and Sadabadi T 2007 J. Iran Chem. Soc. 4 199
Sanz R, Martínez A, Guilarte V, Álvarez-Gutiérrez J M and Rodríguez F 2007 Eur. J. Org. Chem. 28 4642
Lakourag M M, Movassagh B and Fasihi 2000 J. Synth. Commun. 30 821
Tamaddon F, Khoobi M and Keshavarz E 2007 Tetrahedron Lett. 48 3643
Firouzabadi H, Iranpoor N and Khoshnood A 2007 Catal. Commun. 9 529
Yadav J S, Subba Reddy B V, Pandurangam T, Jayasudan Reddy Y and Gupta M K 2007 Catal. Commun. 2 1547
Niknam K and Saberi D 2009 Tetrahedron Lett. 50 5210
Niknam K, Saberi D, Molaee H and Zolfigol M A 2010 Can. J. Chem. 88 164
Polshettiwar V and Varma R S 2008 Tetrahedron Lett. 49 2661
Booth B L, Jibodu K O and Proenca F J R P 1983 J. Chem. Soc. Perkin Trans. 1 1067
Martinez A G, Alvarez R M, Vilar E T, Fraile A G, Hanack M and Subramanian L R 1989 Tetrahedron Lett. 30 581
Acknowledgements
The first author would like to especially thank Dr. E Zia Hosseinipour, Mr. Roozbeh Daneshvar and also Mrs. Leila Hasheminik for their help and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SHAKERI, MS., TAJIK, H. & NIKNAM, K. Preparation of different amides via Ritter reaction from alcohols and nitriles in the presence of silica-bonded N- propyl sulphamic acid (SBNPSA) under solvent-free conditions. J Chem Sci 124, 1025–1032 (2012). https://doi.org/10.1007/s12039-012-0309-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-012-0309-2