Abstract
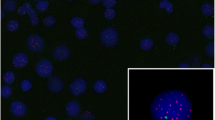
Addition of telomeres to the ends of broken chromosomes has been observed in many malignant cells through the capture of the ends of other chromosomes as a result of nonreciprocal translocations. In this study, we aimed to evaluate the percentage of nuclei with telomere capture (TC%) as a prognostic marker in myelodysplastic syndromes (MDS) patients. This study included 45 newly diagnosed MDS patients, 36 cases with denovo MDS and 9 cases with therapy-related MDS, and another 35 apparently healthy volunteers as a control group. Telomere capture percentage was investigated with fluorescent in situ hybridization technique using a probe for 15qter. We found that median TC% rate was significantly increased in those with bad cytogenetic abnormalities, patients with blast cells >10 % in BM, and patients categorized as high risk according to WHO and IPSS classification; also, there was a significant negative correlation with progression-free survival. Telomere capture serves as a useful marker for the assessment of MDS patient’s risk, and also it had a clinical importance for the early detection of disease progression.


Similar content being viewed by others
References
Béné MC, Feuillard J, Hussonc B, Maynadiéd M. Immunophenotyping of myelodysplasia. Clin Appl Immunol Rev. 2005;5:133–48.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–99.
Greenberg P, Cox C, Lebeau MM, Fenaux P, Morel P, Jacobs RA, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–86.
Pfeilstocker M, Reisner R, Nosslinger T, Gruner H, Nowotny H, Tuchler H. Cross-validation of prognostic scores in myelodysplastic syndromes on 386 patients from a single institution confirms importance of cytogenetics. Br J Haematol. 1999;106:455–63.
Germing U, Strupp C, Kuendgen A, Isa S, Knipp S, Hildebrandt B, Giagounidis A, Aul C, Gattermann N, Haas R. Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Haematologica. 2006;91:1596–604.
Cesana C, Klersy C, Brando B, Nosari A, Scarpati B, Scampini L, et al. Prognostic value of circulating CD34+ cells in myelodysplastic syndromes. Leuk Res. 2008;32:1715–21.
Meltzer PS, Guan X, Trent JM. Telomere capture stabilizes chromosome breakage. Nat Genet. 1993;4:252–5.
Iwama H, Tauchi T, Ohyashiki K, Ohyashiki JH, Hayashi S, Yahata N, Ando K, Toyama K, Hoshika A, Takasaki M, MoriShay JW. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998;102:397–402.
Wilkie AOM, Lamb J, Harris PC, Finney RD, Higgs DR. A truncated human chromosome 16 associated with a thalassaemia is stabilized by addition of telomeric repeat (TTAGGG). Nature. 1990;346:868–71.
Flint J, Craddock CF, Villegas A, Bentley DP, Williams HJ, Galanello R, Cao A, Wood WG, Ayyub H, Higgs DR. Healing of broken human chromosomes by the addition of telomeric repeats. Am J Hum Genet. 1994;55:505–12.
Ning Y, Liang JC, Nagarajan I, Schröck E, Ried T. Characterizationof 5q deletions by subtelomeric probes and spectral karyotyping. Cancer Genet Cytogenet. 1998;103:170–6.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House. Hematol J. 2000;1:53–66.
Stampfer M, Yaswen P. Culture models of human mammary epithelial cell transformation. J Mammary Gland Biol Neoplasia. 2000;5:365–78.
Brown WRA, MacKinnon PJ, Villasante A, Spurr N, Buckle VJ, Dobson MJ. Structure and polymorphism of human telomere associated DNA. Cell. 1990;63:119–32.
Ballif BC, Kashork CD, Shaffer LG. Mechanisms of cytogenetically defined terminal deletions using chromosome-specific subtelomeric probes. Eur J Hum Genet. 2000;8:764–70.
Serakinci N, Ostergaard M, Larsen H, Madsen B, Pedersen B, Koch J. Multiple telomeric aberrations in a telomerase-positive leukemia patient. Cancer Genet Cytogenet. 2002;138:11–6.
Rasnick D, Duesberg P. How aneuploidy affects metabolic control and causes cancer. Biochem J. 1999;340:621–30.
Amiel A, Goldzak G, Gaber E, Yosef G, Fejgin MD, Yukla M, Lishner M. Random aneuploidy and telomere capture in chronic lymphocytic leukemia and chronic myeloid leukemia patients. Genet Cytogenet. 2005;163:12–6.
Bittman LG, Amiel A, Hadary R, Fejgin MD, Quitt M, Cohen YK. Telomere capture in hepatitis C infection. Cancer Genet Cytogenet. 2009;191:63–6.
Ohyashiki JH, Iwama H, Yahata N, Ando K, Hayashi S, Shay JW, Ohyashiki K. Telomere stability is frequently impaired in high groups of patients with myelodysplastic syndromes. Clin Cancer Res. 1999;5:1155–60.
Johansson B, Mertens F, Mitelman F. Primary vs. secondary neoplasia-associated chromosomal abnormalities—balanced rearrangements vs genomic imbalances? Genes Chromosomes Cancer. 1996;16:155–63.
Sieglová Z, Žilovcová S, Cermák J, Ríhová H, Březinová D, Dvořáková R, Marková M, Maaloufová J, Sajdová J, Březinová J, Zemanová Z, Michalová K. Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis. Leuk Res. 2004;29:1013–21.
Slugboom PE, Droog S, Boomsma GI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1993;52:661–7.
Acknowledgments
We gratefully thank all participating subjects for cooperation and support to this study. We also acknowledge Prof. Maha M Atfy for her assistance and guidance.
Conflict of interest
The authors have no conflicts of interest or funding support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labib, H.A., Elshorbagy, S. & Elantonuy, N.G. Significance of telomere capture in myelodysplastic syndromes. Med Oncol 31, 216 (2014). https://doi.org/10.1007/s12032-014-0216-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0216-0