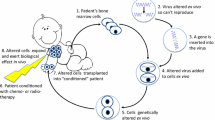
Abstract
The use of gene therapy in the treatment of primary immune deficiencies (PID) has advanced significantly in the last decade. Clinical trials for X-linked severe combined immunodeficiency, adenosine deaminase deficiency (ADA), chronic granulomatous disease, and Wiskott-Aldrich syndrome have demonstrated that gene transfer into hematopoietic stem cells and autologous transplant can result in clinical improvement and is curative for many patients. Unfortunately, early clinical trials were complicated by vector-related insertional mutagenic events for several diseases with the exception of ADA-deficiency SCID. These results prompted the current wave of clinical trials for primary immunodeficiency using alternative retro- or lenti-viral vector constructs that are self-inactivating, and they have shown clinical efficacy without leukemic events thus far. The field of gene therapy continues to progress, with improvements in viral vector profiles, stem cell culturing techniques, and site-specific genome editing platforms. The future of gene therapy is promising, and we are quickly moving towards a time when it will be a standard cellular therapy for many forms of PID.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bousfiha A, Jeddane L, Al-Herz W, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2015;35:727–38. doi:10.1007/s10875-015-0198-5.
Pai S-Y, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–46. doi:10.1056/NEJMoa1401177. A major retrospective study of the factors influencing outcomes for patients with SCID treated by allogeneic transplant in multiple major centers across North America, highlighting the benefits of early diagnosis and treatment.
Gennery AR, Slatter MA, Grandin L, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–610.e11. doi:10.1016/j.jaci.2010.06.015.
Blaese RM, Culver KW, Miller AD, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270(5235):475–80. doi:10.1126/science.270.5235.475.
Chan B, Wara D, Bastian J, et al. Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol. 2005;117(2):133–43. doi:10.1016/j.clim.2005.07.006.
Serana F, Sottini A, Chiarini M, et al. The different extent of B and T cell immune reconstitution after hematopoietic stem cell transplantation and enzyme replacement therapies in SCID patients with adenosine deaminase deficiency. J Immunol. 2010;185(12):7713–22. doi:10.4049/jimmunol.1001770.
Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009;114(17):3524–32. doi:10.1182/blood-2009-06-189209.
Onodera M, Ariga T, Kawamura N, et al. Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood. 1998;91:30–6.
Kohn DB, Weinberg KI, Nolta JA, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1(10):1017–23.
Bordignon C, Mavilio F, Ferrari G, et al. Transfer of the ADA gene into bone marrow cells and peripheral blood lymphocytes for the treatment of patients affected by ADA-deficient SCID. Hum Gene Ther. 1993;4:513–20.
Hoogerbrugge P, van Beusechem V, Fischer A, et al. Bone marrow gene transfer in three patients with adenosine deaminase deficiency. Gene Ther. 1996;3(2):179–83.
Aiuti A PhD, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58. doi:10.1056/NEJMoa0805817. Report of the outcomes for the first 10 ADA-SCID patients treated by gene therapy using a gamma-retroviral vector and reduced intensity conditioning with a high rate of immune reconstitution.
Gaspar HB, Buckland K, Rivat C, et al. Immunological and metabolic correction after lentiviral vector mediated haematopoietic stem cell gene therapy for ADA deficiency. Mol Ther. 2014;22(Supplement 1):S106.
Candotti F, Shaw KL, Muul L, et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency : clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120(18):3635–46. doi:10.1182/blood-2012-02-400937.There.
Carbonaro Sarracino D, Shaw K, Sokolic R, et al. U.S. clinical gene therapy trials for adenosine deaminase-deficienct severe combined immune deficiency (ADA-SCID). J Clin Immunol. 2014;34(S2):139–515. doi:10.1007/s10875-014-0101-9.
Aiuti A, Cassani B, Andolfi G, et al. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J Clin Invest. 2007;117(8):2233–40. doi:10.1172/JCI31666.
Pike-Overzet K, de Ridder D, Weerkamp F, et al. Gene therapy: is IL2RG oncogenic in T-cell development? Nature. 2006;443(7109):E5. doi:10.1038/nature05218. discussion E6-E7.
Sokolic R, Maric I, Kesserwan C, et al. Myeloid dysplasia and bone marrow hypocellularity in adenosine deaminase-deficient severe combined immune deficiency. Blood. 2011;118(10):2688–94. doi:10.1182/blood-2011-01-329359.
Carbonaro Sarracino DA, Jin X, Wang X, et al. Gene therapy/bone marrow transplantation in ADA-deficient mice: roles of enzyme-replacement therapy and cytoreduction. Blood. 2012;120(18):3677–87. doi:10.1182/blood-2012-02-408591.
Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–57. doi:10.1016/0092-8674(93)90167-O.
Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–64. doi:10.1056/NEJMoa1000164. Report of outcomes for subjects treated with a gamma-retroviral vector without any conditioning, leading to restoration of T cell immunity (although several developed T leukoprioliferative complications).
Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364(9452):2181–7. doi:10.1016/S0140-6736(04)17590-9.
Howe SJ, Mansour MR, Schwarzwaelder K, Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Investig. 2008;44(0):1–22. doi:10.1172/JCI35798DS1.
Hacein-Bey Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–42. doi:10.1172/JCI35700. A detailed and sophisticated molecular analysis of the mechanisms of insetrtional oncogenesis leading to T lymphoproliferation in XSCID subjects undergoing gene therapy with a "first generation" gamma-retroviral vector.
Hacein-Bey-Abina S, Pai S-Y, Gaspar HB, et al. A Modified γ-Retrovirus Vector for X-Linked Severe Combined Immunodeficiency. N Engl J Med. 2014;371(15):1407–17. doi:10.1056/NEJMoa1404588. A recent report of successful restoration of T lymphocyte immunity without leukoproliferation or clonal expansions, using a "second generation: gamma-retroviral vector lacking strong enhancer elements.
De Ravin SS, Gray JT, Throm RE, et al. False-positive HIV PCR test following ex vivo lentiviral gene transfer treatment of X-linked severe combined immunodeficiency vector. Mol Ther. 2014;22(2):244–5. doi:10.1038/mt.2013.296.
Seger RA, Gungor T, Belohradsky BH, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood. 2002;100(13):4344–50. doi:10.1182/blood-2002-02-0583. A multi-center trial of reduced intensity conditioning for allogeneic transplant of Chronic Granulomatous Disease with excellent survival, even with unrelated donors.
Kang EM, Choi U, Theobald N, et al. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010;115(4):783–91. doi:10.1182/blood-2009-05-222760. This clinical trial of gene therapoy for CGD using a gamma-retroviral vector achieved only low level engraftment of gene corrected stem cells, yet still provided some benefit to subjects, providing proof-of-principle for this approach.
Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. 2008.
Moratto D, Giliani S, Bonfim C, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an internationalcollaborative study. Blood. 2011;118(6):1675–85. doi:10.1182/blood-2010-11-319376.
Boztug K, Banerjee PP, Ph D, et al. Stem-cell gene therapy for the Wiskott-Aldrich Syndrome. N Engl J Med. 2010;363(20):1918–27.
Braun CJ, Boztug K, Paruzynski A, et al. Gene therapy for wiskott-Aldrich syndrome—long-term efficacy and genotoxicity. Sci Transl Med. 2014;6(227):1–14. doi:10.1126/scitranslmed.3007280.
Hacein-Bey Abina S, Gaspar HB, Blondeau J, et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich Syndrome. JAMA. 2015;313(15):1550. doi:10.1001/jama.2015.3253. Successful restoration of immunity, with partial improvement in platelet counts, by gene therapy for Wiskott-Aldrich Syndrome using a lentiviral vector.
Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich Syndrome. Science. 2013;341(6148):1–12. doi:10.1126/science.1233151. Successful restoration of immunity, with partial improvement in platelet counts, by gene therapy for Wiskott-Aldrich Syndrome using a lentiviral vector.
Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10(2):218–29. doi:10.1016/j.stem.2012.01.003.
Pu J, Frescas D, Zhang B, Feng J. Utilization of TALEN and CRISPR/Cas9 technologies for gene targeting and modification. Exp Biol Med. 2015;240(8):1065–70. doi:10.1177/1535370215584932.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Kuo and Kohn declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Immune Deficiency and Dysregulation
Rights and permissions
About this article
Cite this article
Kuo, C.Y., Kohn, D.B. Gene Therapy for the Treatment of Primary Immune Deficiencies. Curr Allergy Asthma Rep 16, 39 (2016). https://doi.org/10.1007/s11882-016-0615-8
Published:
DOI: https://doi.org/10.1007/s11882-016-0615-8