Abstract
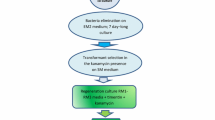
A procedure for regenerating plants of Lupinus mutabilis from shoot apices, from which the leaf primordia and initial cell layer(s) of the apical meristem were removed, has been used to generate transgenic plants following Agrobacterium tumefaciens-mediated gene delivery. Transformation competent cells, from which buds developed, were located at the periphery of the apical meristem. Kanamycin resistant plants were obtained which expressed β-glucuronidase activity. Integration of the neomycin phosphotransferase II and β-glucuronidase genes into the genomes of transgenic plants was confirmed by non-radioactive DNA-DNA hybridisation. This is the first report of the generation of transgenic plants in L. mutabilis.
Similar content being viewed by others
Abbreviations
- BAP:
-
6-benzylamino purine
- bar :
-
bialaphos (phosphinothricin) resistance gene
- CPPU:
-
N-(2-chloro-4-pyridyl)-N′-phenylurea
- DAS:
-
Double Antibody Sandwich
- GUS:
-
β-glucuronidase
- IBA:
-
indole-3-butyric acid
- MS:
-
Murashige and Skoog (1962)
- 4-MU:
-
4-methylumbelliferyl glucuronide
- NAA:
-
α-naphthaleneacetic acid
- NPTII:
-
neomycin phosphotransferase
- X-Gluc:
-
5-bromo-4-chloro-3-indolyl-β-D-glucuronide
References
Berlin J., Fecker L., Rugenhagen C., Sator C., Strack D., Witte L., Wray V. 1991a. Isoflavone glycoside formation in transformed and non-transformed suspension and hairy root cultures of L. polyphyllus and L. hartwegii. Z. Naturforsch. 46c: 725–734.
Berlin J., Rugenhagen C., Rippert M., Erdogan S. 1991b. Effects of culture conditions on isoflavonoid levels of transformed and non-transformed cultures of Lupinus — A comparison of suspension and hairy root cultures. Z. Naturforsch. 46c: 735–742.
Berlyn G.P., Miksche J.P. 1976. Botanical microtechnique and cytochemistry. Iowa State University Press, Ames, Iowa, pp 30–65.
Bradford M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72: 248–254.
Christou P. 1992. Genetic engineering and in vitro culture of crop legumes. Technomic Publishing Co. Inc., Lancaster, USA.
Curtis I.S., Power J.B., Blackhall N.W., de Laat A.M.M., Davey M.R. 1994. Genotype-independent transformation of lettuce using Agrobacterium tumefaciens. J. Exp. Bot. 45: 1441–1449.
Dellaporta S.L., Wood J., Hicks J.B. 1983. A plant DNA minipreparation: version II. Plant Mol. Biol. Reporter 4: 9–21.
Fontana G.S., Santini L., Caretto S., Frugis G., Mariotti D. 1993. Genetic transformation in the grain legume Cicer arietinum L. (Chickpea). Plant Cell Rep. 12: 194–198.
Fromm M.E., Taylor L.P., Walbot V. 1986. Stable transformation of maize after gene transfer by electroporation. Nature 319: 791–793.
Gamborg O.L., Miller R.A., Ojima K. 1968. Nutrition requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 50: 151–159.
Gartland K.M.A., Phillips J.P., Vitha S., Benes K. 1995. Fluorometric GUS analysis for transformed plant material. In: Methods in Molecular Biology Vol 44. Agrobacterium Protocols, ed. by K.M.A. Gartland, M.R. Davey, Humana Press Publ., Totowa, USA: 195–199.
Gladstones J.S. 1984. Present situation and potential of Mediterranean/African lupins for crop production. In: Proc. Third Internatl. Lupin Conf., La Rochella, France: 18–37.
Hussey G., Johnson R.D., Warren S. 1989. Transformation of meristematic cells in the shoot apex of cultured pea shoots by Agrobacterium tumefaciens and A. rhizogenes. Protoplasma 148: 101–105.
Jacobsen H.J. 1992. Biotechnology applied to grain legumes — current state and prospects. In: Proc. 1st European Conf. on Grain Legumes, Angers, France: 99–103.
Jefferson R.A., Kavanagh T.A., Bevan M.W. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907.
Jin S., Komari T., Gordon M.P., Nester E.W. 1987. Genes responsible for the supervirulence phenotype of Agrobacterium tumefaciens A281. J. Bacteriol. 169: 4417–4425.
Kathen A. de, Jacobsen H.J. 1995. Cell competence for Agrobacterium-mediated DNA transfer in Pisum sativum L. Transgenic Res. 4: 184–191.
Lion T., Haas O.A. 1990. Nonradioactive labeling of probe with digoxigenin by polymerase chain reaction. Anal. Biochem. 188: 335–337.
Marchant R., Davey M.R., Lucas J.A., Lamb C.J., Dixon R.A., Power J.B. 1998. Expression of a chitinase transgene in rose (Rosa hybrida L.) reduces development of blackspot disease (Diplocarpon rosae Wolf). Molec. Breed. 4: 187–194.
McCabe M.S., Power J.B., de Laat A.M.M., Davey M.R. 1996. Detection of single copy genes in DNA from transgenic plants by non-radioactive Southern blot analysis. Mol. Biotechnol. 7: 1–6.
Medford J.I. 1992. Vegetative apical meristems. The Plant Cell 4: 1029–1039.
Micallef M.C., Austin S., Bingham E.T. 1995. Improvement of transgenic alfalfa by backcrossing. In Vitro Cell Dev. Biol.-Plant 131: 187–192.
Molvig L., Tabe L.M., Eggum B.O., Moore A.C., Craig S., Spencer D., Higgins T.J.V. 1997. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc. Natl. Acad. Sci. USA 94: 8393–8398.
Murashige T., Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Nadolska-Orczyk A. 1992. Somatic embryogenesis of agriculturally important lupin species (Lupinus angustifolius, L. alba, L. mutabilis). Plant Cell Tiss. Org. Cult. 28: 19–25.
Nauerby B., Madsen M., Christiansen J., Wyndaele R. 1991. A rapid and efficient regeneration system for pea (Pisum sativum), suitable for transformation. Plant Cell Rep. 9: 676–697.
Phoplonker M.A., Caligari P.D.S. 1992. Callus formation and plant regeneration in Lupinus mutabilis. In: Proc Ist European Conf. on Grain Legumes, Angers, France: 109–110.
Pigeaire A., Abernethy D., Smith P.M., Simpson K., Fletcher N., Lu C.Y., Atkins C.A., Cornish E. 1997. Transformation of a grain legume (Lupinus angustifolius L.) via Agrobacterium tumefaciens-mediated gene transfer to shoot apices. Molec. Breed. 3: 341–349.
Pythoud F., Sinkar V.P., Nester E.W., Gordon M.P. 1987. Increased virulence of Agrobacterium rhizogenes conferred by the vir region of pTiBo542: application to genetic engineering of poplar. Bio/Technol. 5: 1323–1327.
Römer P., Jahn-Deesbach W. 1988. Developments in Lupinus mutabilis breeding. In: Proc. Vth Internatl. Lupin Conf., Poznan, Poland: 41–50.
Römer P., Weissmann E. 1990. Perspectives of practical lupin breeding. In: Proc VIth Internatl. Lupin Conf., Temuco, Chile: 350–362.
Saalbach I., Pickardt T., Machemehl F., Saalbach G., Schieder O., Muntz K. 1994. A chimeric gene encoding the methionine-rich 2S albumin of the Brazil nut (Bertolletia excelsa H.B.K.) is stably expressed and inherited in transgenic grain legumes. Mol. Gen. Genet. 242: 226–236.
Sator C. 1990. Lupins (Lupinus spp.). In: Biotechnology in Agriculture and Forestry. Legumes and Oilseed Crops I, Vol 10, ed. By Y.P.S. Bajaj, Springer-Verlag Publ., Heidelberg: 288–311.
Schroeder H.E., Gollasch S., Moore A., Tabe L.M., Craig S., Hardie D.C., Chrispeels M.J., Spencer D., Higgins T.J.V. 1995. Bean α-amylase confers resistance to the Pea Weevil (Bruchus pisorum) in transgenic peas (Pisum sativum L.). Plant Physiol. 107: 1233–1239.
Somsap V., Cooper J.I., Li D., Jones M.G.K. 1994. Tissue culture and transformation of lupins. In: Abstracts VIIIth Internatl. Congr. Plant Tiss. and Cell Cult., Firenze, Italy: S1–25.
Sroga G.E. 1987. Plant regeneration of two Lupinus spp. from callus cultures via organogenesis. Plant Sci. 51: 245–249.
Taylor B.H., Amasino R.M., White F.F., Nester E.W., Gordon M.P. 1985. T-DNA analysis of plants regenerated from hairy root tumours. Mol. Gen. Genet. 201: 554–557.
Vancanneyt G., Schmidt R., O’Connor-Sanchez A., Willmitzer L., Rosa-Sosa M. 1990. Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. 220: 245–250.
Warkentin T.D., McHughen A. 1992. Agrobacterium tumefaciens-mediated beta-glucuronidase (GUS) gene expression in lentil (Lens culinaris Medik.) tissues. Plant Cell Rep. 11: 274–278.
Werbrouck S.P.O., Debergh P.C. 1994. Applied aspects of plant regeneration. In: Plant Cell Culture: A Practical Approach. Second Editn, ed. by R.A. Dixon, R.A. Gonzales, IRL Oxford University Press Publ., Oxford, UK: 127–135.
Wink M. 1988. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75: 225–233.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Babaoglu, M., McCabe, M.S., Power, J.B. et al. Agrobacterium-mediated transformation of Lupinus mutabilis L. using shoot apical explants. Acta Physiol Plant 22, 111–119 (2000). https://doi.org/10.1007/s11738-000-0064-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-000-0064-8