Abstract
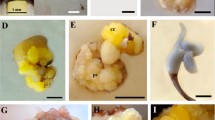
Using immature embryos and cotyledons as explants, a successful system to culture immature embryos and induce direct regeneration from cotyledons was established for Prunus mume “Xuemei”. For immature embryo culture, a high frequency of plantlet formation (89.5%) from the embryonic axis was obtained using half-strength Murashige and Skoog (1/2 MS) medium supplemented with 13.2 μM 6-benzyladenine (BA) and 2.7 μM 1-naphthaleneacetic (NAA). Shoots formed directly from cotyledons with the embryo axis intact when explants were cultured on 1/2 MS medium containing 2.2 μM BA with different combinations of NAA (2.7, 5.4 μM) and indole-3-butyric acid (IBA) (0, 2.5, 5.0 μM). Better results were achieved when the embryonic axis was removed from the cotyledons and cultured on 1/2 MS medium supplement with 13.2 μM BA, 2.7 μM NAA or 2.2 μM BA, 2.2 μM thidiazuron (TDZ), and 2.7 μM NAA, respectively. Regenerated shoots were successfully rooted on 1/2 MS or Woody Plant medium (WPM) supplemented with 2.5–5.0 μM IBA. The effect of the embryonic axis, BA, and TDZ on cotyledon regeneration was investigated in detail. Rooted plantlets were transferred to soil successfully.

Similar content being viewed by others
References
Antonelli, M. Regeneration from almond cotyledons: induction of proembryonal masses. Acta Hortic. 300: 255–259; 1991.
Bhagwat, B.; David, L. W. In vitro shoot regeneration from leaves of sweet cherry (Prunus avium) ‘Lapins’ and ‘Sweetheart’. Plant Cell Tissue Organ Cult. 78: 173–181; 2004.
Chen, J. Y. Prunus mume in China. Haikou, China: Hainan Publishing House; 1997.
Declerck, V.; Korban, S. S. Influence of growth regulators and carbon sources on callus induction, growth and morphogenesis from leaf tissues of peach (Prunus persica L. Batsch). J. Hort. Sci. 71:49–55; 1996.
Dogasaki, C.; Kakuno, Y.; Honda, M.; Takada, N.; Maruyama, T.; Nishijima, M.; Adachi, Y.; Ohno, N.; Yadomae, T.; Miyazaki, T. Contribution to immunochemical analysis of polysaccharides in medicinal plants. Drug Design Rev. 1: 153–159; 2004.
Escalettes, V.; Dosba, F. In vitro adventitious shoot regeneration from leaves of Prunus spp. Plant Sci. 90: 201–209; 1993.
Goffreda, J. C.; Scopel, A. L.; Fiola, J. A. Indole butyric acid induces regeneration of phenotypically normal apricot (Prunus armeniaca L.) plants from immature embryos. Plant Growth Regul. 17: 41–46; 1995.
Han, J. S.; Oh, D. G.; Mok, I. G.; Park, H. G.; Kim, C. K. Efficient plant regeneration from cotyledon explants of bottle gourd (Lagenaria siceraria Standl). Plant Cell Rep. 23: 291–296; 2004.
Harada, H. H.; Murai, Y. Micropropagation of Prunus mume. Plant Cell Tissue Organ Cult. 46: 265–267; 1996.
Hokanson, K. E.; Pooler, M. R. Regeneration of ornamental cherry (Prunus) taxa from mature stored seed. HortScience. 35: 745–748; 2000.
Katoka, I. Interspecific hybridization between Microcerasus and Prunus spp. J. Jpn. Soc. Hort. Sci. 4: 398–407; 1988.
Kouider, M.; Korban, S. S.; Skirvin, R. M.; Chu, M. N. Influence of embryonic dominance and polarity on adventitious shoot formation from apple cotyledons in vitro. J. Am. Soc. Hort. Sci. 109: 381–385; 1984.
Lane, W. D.; Cossio, F. Adventitious shoots from cotyledons of immature cherry and apricot embryos. Can. J. Plant Sci. 66: 953–959; 1986.
Lloyd, G. B.; McCown, B. H. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant. Prop. Soc. 30: 421–427; 1980.
Mante, S. R.; Scorza, R.; Cordts, J. M. Plant regeneration from cotyledons of Prunus persica, Prunus domestica and Prunus cerasus. Plant Cell Tissue Organ Cult. 19: 1–11; 1989.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962.
Pellegrineschi, A.; Fatokun, C. A.; Thottappilly, G.; Adepoju, A. Cowpea embryo rescue.1. Influence of culture medium composition on plant recovery from isolated immature embryos. Plant Cell Rep. 17: 133–138; 1997.
Pooler, M. R.; Scorza, R. Regeneration of peach (Prunus persica (L.) Batsch) rootstock cultivars from cotyledons of mature stored seed. HortScience. 30: 355–356; 1995.
Salajova, T.; Salaj, J. Somatic embryogenesis and plantlet regeneration from cotyledon explants isolated from embryos and seedlings of hybrid fir. J. Plant Physiol. 158: 747–755; 2001.
Simon, H.; Raharjo, T.; Litz, R. E. Micrografting and ex vitro grafting for somatic embryo rescue and plantrecovery in avocado (Persea americana). Plant Cell Tissue Organ Cult. 82: 1–9; 2005.
Singh, A. K; Suresh, C. Somatic embryogenesis and plant regeneration from cotyledon explants of a timber-yielding leguminous tree, Dalbergia sissoo Roxb. J. Plant Physiol. 160: 415–421; 2003.
Viloria, Z.; Grosser, J. W.; Bracho, B. Immature embryo rescue, culture and seedling development of acid citrus fruit derived from interploid hybridization. Plant Cell Tissue Organ Cult. 82: 159–167; 2005.
Acknowledgments
We thank all the colleagues in our lab for constructive discussion and technical support, and are also grateful to all the staff of Wuhan P. mume garden, China for providing experimental materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: F. Hammerschlag
Rights and permissions
About this article
Cite this article
Ning, G.G., Bai, S.P., Bao, M.Z. et al. Factors affecting plantlet regeneration from in vitro cultured immature embryos and cotyledons of Prunus mume “Xue mei”. In Vitro Cell.Dev.Biol.-Plant 43, 95–100 (2007). https://doi.org/10.1007/s11627-007-9035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-007-9035-8