Abstract
Purpose
The aim of this study was to compare [11C]Pittsburgh compound B ([11C]PiB) and [18F]florbetaben ([18F]FBB) for preclinical investigations of amyloid-β pathology.
Procedures
We investigated two aged animal models of cerebral amyloidosis with contrasting levels of amyloid-β relating to “high” (APPPS1-21 n = 6, wild type (WT) n = 7) and “low” (BRI1-42 n = 6, WT n = 6) target states, respectively.
Results
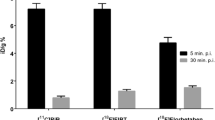
APPPS1-21 mice (high target state) demonstrated extensive fibrillar amyloid-β deposition that translated to significantly increased retention of [11C]PiB and [18F]FBB in comparison to their wild type. The retention pattern of [11C]PiB and [18F]FBB in this cohort displayed a significant correlation. However, the relative difference in tracer uptake between diseased and healthy mice was substantially higher for [11C]PiB than for [18F]FBB. Although immunohistochemistry confirmed the high plaque load in APPPS1-21 mice, correlation between tracer uptake and ex vivo quantification of amyloid-β was poor for both tracers. BRI1-42 mice (low target state) did not demonstrate increased tracer uptake.
Conclusions
In cases of high fibrillar amyloid-β burden, both tracers detected significant differences between diseased and healthy mice, with [11C]PiB showing a larger dynamic range.






Similar content being viewed by others
References
Selkoe DJ (1991) The molecular pathology of Alzheimer’s. Neuron 6:487–498
Nordberg A (2011) Molecular imaging in Alzheimer’s disease: new perspectives on biomarkers for early diagnosis and drug development. Alzheimers Res Ther 3:34
Reiman EM, Jagust WJ (2012) Brain imaging in the study of Alzheimer’s disease. Neuroimage 61:505–516
Klunk WE, Engler H, Nordberg A et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol 55:306–319
Mintun MA, Larossa GN, Sheline YI et al (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67:446–452
Manook A, Yousefi BH, Willuweit A et al (2012) Small-animal PET imaging of amyloid-beta plaques with [11C] PiB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer’s disease. PLoS One 7:e31310
Maeda J, Ji B, Irie T et al (2007) Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J Neurosci 27:10957–10968
von Reutern B, Grünecker B, Yousefi BH, et al. (2013) Voxel-based analysis of amyloid-burden measured with [11C]PiB PET in a double transgenic mouse model of Alzheimer’s disease. Mol Imaging Biol
Nelissen N, Van Laere K, Thurfjell L et al (2009) Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med 50:1251–1259
Rowe CC, Ellis KA, Rimajova M et al (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31:1275–1283
Wong DF, Moghekar AR, Rigamonti D et al (2013) An in vivo evaluation of cerebral cortical amyloid with [18F]flutemetamol using positron emission tomography compared with parietal biopsy samples in living normal pressure hydrocephalus patients. Mol Imaging Biol 15:230–237
Rowe CC, Ackerman U, Browne W et al (2008) Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 7:129–135
Clark CM, Pontecorvo MJ, Beach TG et al (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 11:669–678
Wong DF, Rosenberg PB, Zhou Y et al (2010) In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med 51:913–920
Cselenyi Z, Jonhagen ME, Forsberg A et al (2012) Clinical validation of 18F-AZD4694, an amyloid-b -specific PET radioligand. J Nucl Med 53:415–424
Ni R, Gillberg PG, Bergfors A et al (2013) Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain 136:2217–2227
Snellman A, Lopez-Picon FR, Rokka J et al (2013) Longitudinal amyloid imaging in mouse brain with 11C-PIB: comparison of APP23, Tg2576, and APPswe-PS1dE9 mouse models of Alzheimer disease. J Nucl Med 54:1434–1441
Poisnel GG, Dhilly MM, Moustié OO et al (2012) PET imaging with [18F]AV-45 in an APP/PS1-21 murine model of amyloid plaque deposition. Neurobiol Aging 33:2561–2571
Rominger A, Brendel M, Burgold S et al (2013) Longitudinal assessment of cerebral beta-Amyloid deposition in mice overexpressing Swedish mutant beta-amyloid precursor protein using 18F-Florbetaben PET. J Nucl Med 54:1127–1134
Kepe V, Moghbel MC, Långström B et al (2013) Amyloid-β positron emission tomography imaging probes: a critical review. J Alzheimers Dis 36:613–631
Radde R, Bolmont T, Kaeser SA et al (2006) Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7:940–946
McGowan E, Pickford F, Kim J et al (2005) Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47:191–199
Levites Y, Smithson LA, Price RW et al (2006) Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer’s disease mouse models. FASEB J 20:2576–2578
Hudson HM, Larkin RS (1994) Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 13:601–609
Defrise M, Kinahan PE, Townsend DW et al (1997) Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging 16:145–158
Watson CC (2000) New, faster, image-based scatter correction for 3D PET. IEEE Trans Nucl Sci 47:1587–1594
Mirrione MM, Schiffer WK, Fowler JS et al (2007) A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage 38:34–42
Franklin KB, Paxinos G (1997) Mouse brain in stereotaxic coordinates
Rojas S, Herance JR, Gispert JD et al (2013) In vivo evaluation of amyloid deposition and brain glucose metabolism of 5XFAD mice using positron emission tomography. Neurobiol Aging 34:1790–1798
Landau SM, Breault C, Joshi AD et al (2013) Amyloid-b imaging with Pittsburgh compound B and Florbetapir: comparing radiotracers and quantification methods. J Nucl Med 54:70–77
Villemagne VL, Mulligan R, Pejoska S et al (2012) Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 39:983–989
Rowe CC, Pejoska S, Mulligan RS et al (2013) Head-to-head comparison of 11C-PiB and 18F-AZD4694 (NAV4694) for b-amyloid imaging in aging and dementia. J Nucl Med 54:880–886
Pike VW (2009) PET radiotracers: crossing the blood–brain barrier and surviving metabolism. Trends Pharmacol Sci 30:431–440
Price JC, Klunk WE, Lopresti BJ et al (2005) Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab 25:1528–1547
Fodero-Tavoletti MT, Rowe CC, McLean CA et al (2009) Characterization of PiB binding to white matter in Alzheimer disease and other dementias. J Nucl Med 50:198–204
Snellman A, Rokka J, Lopez-Picon FR et al (2012) Pharmacokinetics of [18F]flutemetamol in wild-type rodents and its binding to beta amyloid deposits in a mouse model of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 39:1784–1795
Patt M, Schildan A, Barthel H et al (2010) Metabolite analysis of [18F]Florbetaben (BAY 94-9172) in human subjects: a substudy within a proof of mechanism clinical trial. J Radioanal Nucl Chem 284:557–562
Brendel M, Delker A, Rötzer C et al (2014) Impact of partial volume effect correction on cerebral β-amyloid imaging in APP-Swe mice using [(18)F]-florbetaben PET. Neuroimage 84:843–853
Cole GB, Keum G, Liu J et al (2010) Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates. Proc Natl Acad Sci U S A 107:6222–6227
Lockhart A, Lamb JR, Osredkar T et al (2007) PIB is a non-specific imaging marker of amyloid-beta (A) peptide-related cerebral amyloidosis. Brain 130:2607–2615
Kara F, van Dongen ES, Schliebs R et al (2012) Monitoring blood flow alterations in the Tg2576 mouse model of Alzheimer’s disease by in vivo magnetic resonance angiography at 17.6T. Neuroimage 60:958–966
Klunk WE, Lopresti BJ, Ikonomovic MD et al (2005) Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci 25:10598–10606
Toyama H, Ye D, Ichise M et al (2005) PET imaging of brain with the β-amyloid probe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 32:593–600
Maier FC, Wehrl HF, Schmid AM et al (2014) Longitudinal PET-MRI reveals beta-amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion. Nat Med 20:1485–1492
Acknowledgments
The authors are very thankful to Arnold Spaans from the BV Cyclotron VU (Amsterdam, The Netherlands) for providing us with the shipments of [18F]FBB and to Philippe Joye and Joke Parthoens (Molecular Imaging Center Antwerp) for technical support with in vivo experiments. The authors acknowledge the company KOESLER (Rottenburg, 613 Germany) and Dr. Mathias Jucker (University of Tubingen) for providing the APPPS1 mice. The BRI mice were obtained from Dr. Samir Kumar-Singh of the Flanders Interuniversity Institute for Biotechnology (VIB), who originally obtained them from the Mayo Foundation for Medical Education and Research. This work was funded by the Agency for Innovation by Science and Technology (IWT), by Antwerp University and Antwerp University Hospital, Belgium. Experimental resources are supported by a research contract (46/FA02006/WP130001) of Antwerp University and Janssen Pharmaceutica and by the European Union’s Seventh Framework Programme under Grant agreement no. CP-IP 212043-2 (NAD) and no. 278850 (INMiND). The funding sources had no further role in the study design or in the collection, analysis and interpretation of the data.
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldron, AM., Verhaeghe, J., wyffels, L. et al. Preclinical Comparison of the Amyloid-β Radioligands [11C]Pittsburgh compound B and [18F]florbetaben in Aged APPPS1-21 and BRI1-42 Mouse Models of Cerebral Amyloidosis. Mol Imaging Biol 17, 688–696 (2015). https://doi.org/10.1007/s11307-015-0833-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0833-9