Abstract
Purpose
This study employed 3′-deoxy-3′-[18F]-fluorothymidine ([18F]FLT) microPET scanning to assess the treatment response of histone deacetylase inhibitors (HDACi), e.g., N1-hydroxy-N8-phenyloctanediamide (SAHA) and its iodinated derivative ISAHA, in a hepatoma mouse model.
Procedures
The in vitro cytotoxicity of HDACi in various hepatoma cell lines was determined by MTT assay and flow cytometry. ISAHA and SAHA were used to treat HepG2 hepatoma xenograft-bearing mice. The treatment responses were characterized in terms of tumor burden, microPET imaging, and immunohistochemical staining of tumor sections.
Results
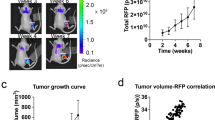
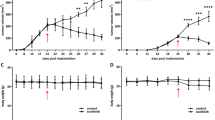
ISAHA effectively inhibited HepG2 hepatoma cell survival and tumor growth. A significantly reduced tumor uptake during HDACi treatment was noticed in [18F]FLT microPET imaging, which was consistent with the findings in immunohistochemical staining.
Conclusions
ISAHA can suppress tumor cell proliferation both in vitro and in vivo. [18F]FLT PET is a promising modality for evaluating the in vivo therapeutic efficacy of HDACi at the early stage of treatment.





Similar content being viewed by others
References
Chen CL, Yang HI, Yang WS et al (2008) Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 135:111–121
Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128:683–692
Sharma S, Kelly TK, Jones PA (2010) Epigenetics in cancer. Carcinogenesis 31:27–36
Yoo CB, Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5:37–50
Sakajiri S, Kumagai T, Kawamata N et al (2005) Histone deacetylase inhibitors profoundly decrease proliferation of human lymphoid cancer cell lines. Exp Hematol 33:53–61
Bernardo MM, Meng Y, Lockett J et al (2011) Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes cancer 2:1009–1022
Lee JH, Choy ML, Ngo L, Venta-Perez G, Marks PA (2011) Role of checkpoint kinase 1 (Chk1) in the mechanisms of resistance to histone deacetylase inhibitors. PNAS 108:19629–19634
Dai Y, Rahmani M, Dent P, Grant S (2005) Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol 25:5429–5444
Qian DZ, Wang X, Kachhap SK et al (2004) The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res 64:6626–6634
Tan J, Cang S, Ma Y, Petrillo RL, Liu D (2010) Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol 3:5
Emanuele S, Lauricella M, Carlisi D et al (2007) SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis 12:1327–1338
Bruzzese F, Leone A, Rocco M et al (2011) HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol 226:2378–2390
Duvic M, Talpur R, Ni X et al (2007) Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109:31–39
Munster PN, Thurn KT, Thomas S et al (2011) A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer 104:1828–1835
Fakih MG, Groman A, McMahon J, Wilding G, Muindi JR (2012) A randomized phase II study of two doses of vorinostat in combination with 5-FU/LV in patients with refractory colorectal cancer. Cancer Chemother Pharmacol 69:743–751
Shields AF (2006) Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. M Mol Imaging Biol 8:141–150
Kenny LM, Vigushin DM, Al-Nahhas A et al (2005) Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18 F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res 65:10104–10112
Boles Ponto LL, Menda Y, Dornfeld K et al (2010) Stability of 3′-deoxy-3′-[18F]fluorothymidine standardized uptake values in head and neck cancer over time. Cancer Biother Radiopharm 25:361–363
Wieder HA, Geinitz H, Rosenberg R et al (2007) PET imaging with [18F]3′-deoxy-3′-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur J Nucl Med Mol Imaging 34:878–883
Wu CY, Chou LS, Chan PC et al (2013) Monitoring tumor response with radiolabeled nucleoside analogs in a hepatoma-bearing mouse model early after doxisome® treatment. Mol Imaging Biol 15:326–335
Leyton J, Alao JP, Da Costa M et al (2006) In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18 F]fluorothymidine positron emission tomography. Cancer Res 66:7621–7629
Na YS, Jung KA, Kim SM et al (2011) The histone deacetylase inhibitor PXD101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models. Cancer Chemother Pharmacol 68:389–398
Stowell JC, Huot RI, Van Voast L (1995) The synthesis of N-hydroxy-N′-phenyloctanediamide and its inhibitory effect on proliferation of AXC rat prostate cancer cells. J Med Chem 38:1411–1413
Kim DW, Ahn DS, Oh YH et al (2006) A new class of SN2 reactions catalyzed by protic solvents: facile fluorination for isotopic labeling of diagnostic molecules. J Am Chem Soc 128:16394–16397
Carlisi D, Vassallo B, Lauricella M et al (2008) Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. Int J Oncol 32:177–184
Duvic M (2008) Histone deacetylase inhibitors: SAHA (Vorinostat). A treatment option for advanced cutaneous T-cell lymphoma. Hematol Meet Rep 2:39–43
Konsoula R, Jung M (2008) In vitro plasma stability, permeability and solubility of mercaptoacetamide histone deacetylase inhibitors. Int J Pharm 361:19–25
Parise RA, Holleran JL, Beumer JH, Ramalingam S, Egorin MJ (2006) A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B 840:108–115
Rubin EH, Agrawal NG, Friedman EJ et al (2006) A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res 12:7039–7045
Suzuki T, Matsuura A, Kouketsu A, Nakagawa H, Miyata N (2005) Identification of a potent non-hydroxamate histone deacetylase inhibitor by mechanism-based drug design. Bioorg Med Chem Lett 15:331–335
Kelly WK, Richon VM, O'Connor O et al (2003) Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 9:3578–3588
Kelly WK, O'Connor OA, Krug LM et al (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Been LB, Suurmeijer AJ, Cobben DC et al (2004) [18 F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging 31:1659–1672
Cheriyath V, Kuhns MA, Kalaycio ME, Borden EC (2011) Potentiation of apoptosis by histone deacetylase inhibitors and doxorubicin combination: cytoplasmic cathepsin B as a mediator of apoptosis in multiple myeloma. Br J Cancer 104:957–967
Acknowledgments
The authors thank the financial support from National Science Council, Taiwan (NSC 101-2623-E-010-006-NU, NSC100-2623-E-010-003-NU, and NSC99-NU-E-010-003). The authors also appreciate the technical support provided by the Taiwan Mouse Clinic, National Comprehensive Mouse Phenotyping and Drug Testing Center, Taipei, Taiwan.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Pei-Chia Chan and Chun-Yi Wu contribute equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 15329 kb)
Rights and permissions
About this article
Cite this article
Chan, PC., Wu, CY., Chou, LS. et al. Monitoring Tumor Response After Histone Deacetylase Inhibitor Treatment Using 3′-Deoxy-3′-[18F]-fluorothymidine PET. Mol Imaging Biol 17, 394–402 (2015). https://doi.org/10.1007/s11307-014-0774-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0774-8