Abstract
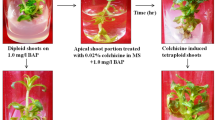
To induce variation through chromosome doubling in Gerbera jamesonii Bolus cv. Sciella, two-week-old in vitro grown shoots were treated with various concentrations of colchicine (0.01, 0.05, 0.10, 0.50 or 1% w/v) for 2, 4 or 8 h. Treated shoots were then cultured on Murashige and Skoog (MS) medium supplemented with 8.8 μM 6-benzyladenine (BA) and 155 μM adenine sulphate (ADS), and subsequently transferred to fresh MS medium containing 2.85 μM indole-3 acetic acid (IAA) for rooting. When shoots were treated with 0.1% colchicine for 8 h, 64% of recovered plantlets were tetraploid. Ploidy of plantlets was confirmed by flow cytometry, stomatal analysis, and morphological characters. Tetraploid plantlets displayed slower proliferation along with higher vigor and thickened broad leaves. Moreover, tetraploid plants developed larger flowers, longer stalks, and have improved vase-life, all contributing to higher ornamental value of gerbera.




Similar content being viewed by others
Abbreviations
- ADS:
-
Adenine sulphate
- BA:
-
6-Benzyladenine
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog (1962)
- PGR:
-
Plant growth regulator
References
Boaventura YMS, Medina DM, Vieira MJFR, Arruda HV (1981) Número de cloroplastos e nĭvel de ploidia em espécies de Coffea L. Rev Bras Bot 4:15–21
Carvalho R, Guerra M, Carvalho PCL (1999) Occurrence of spontaneous triploids in Manihot esculenta Cruntz. Cytologia 64:137–140
Chavadej S, Becker H (1984) Influence of colchicine treatment on chromosome number and growth rate of tissue cultures of Valeriana Wallichii DC. Plant Cell Tiss Organ Cult 3:265–272
Chen WH, Tang CY, Kao YL (2009) Ploidy doubling by in vitro culture of excised protocorms or protocorms-like bodies in Phalaenopsis species. Plant Cell Tiss Organ Cult 98:229–238
Clarke J (1960) Preparation of leaf epidermis for tropographic study. Stain Technol 35:35–39
Cramer CS (1991) Hybridization between diploid and tetraploid Pelargonium × hortorum Bailey. UnderGraduate Honourse Thesis, Pa. State Univ., University Park
De Oliveira VM, Forni-Martins ER, Magalhães PM, Alves MN (2004) Chromosomal and morphological studies of diploid and polyploid cytotypes of Stevia rebaudiana (Bertoni) (Eupatorieae, Asteraceae). Genet Mol Biol 27:215–222
Devadasan K (2008) Diversification and value addition are the key words. The Hindu Survey Ind Agric :97–101
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult 104:329–341
Dickson EE, Arumuganathan K, Koresovich S, Doyle JJ (1992) Nuclear DNA content variation within the Rosaceae. Am J Bot 79:1081–1086
Dolezel J, Greilhuber J, Suda J (2007) Flow cytometry with plants: an overview. In: Dolezel J, Greilhuber J, Suda J (eds) Flow cytometry with plant cells. Wiley, Weinheim, pp 41–65
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Evans AM (1955) The production and identification of polyploids in red clover, white clover and Lucerne. New Phytol 514:149–162
Forni-Martins ER, Cruz ND (1985) Dados preliminaries sobre indução de poliploidia em Jatropha curcas (Euphorbiaceae). Congresso da Sociedade Botànica de São Paulo. V. Botucatu. Resumos, p 167
Galbraith DW, Harkns KR, Maddox JM (1983) Rapid flowcytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051
Gantait S (2009) Accelerated cloning in vitro and induction of variation in six plants having medicinal, aromatic or aesthetic importance. PhD Thesis, Bidhan Chandra Krishi Viswavidyalaya, West Bengal, India
Gu XF, Yang AF, Meng H, Zhang JR (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill cv. Zhanhua. Plant Cell Rep 24:671–676
Han L, Yan B, Zhang T, Jiang Y, Zhang H, Yu L, Li S (2009) Preliminary studies on polyploidy mutation of cut flower Gerbera jamesonii Bolus. Acta Hortic Sinica 36:605–610
Honkanen J, Aapola A, Seppänen P, Törmälä T, Oy K, de Wit JC, Esendam HF, Stravers LJM, Terra Nigra BV (1991) Production of double haploid gerbera clones. Acta Hortic 300:341–346
Kang XY (2003) Advances in researches on polyploid breeding of forest trees. J Beijing For Univ 25:70–74
Kanwar JK, Kumar S (2008) In vitro propagation of Gerbera—a review. Hort Sci 35:35–44
Leblanc O, Hernández M, Bello S, Garcia V, Bertaud J, Savidan Y (1995) Chromosome doubling in Tripsacum: the production of artificial, sexual tetraploid plants. Plant Breed 114:226–230
Liu G, Li Z, Bao M (2007) Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 157:145–154
Lyrene PM, Perry JL (1982) Production and selection of blueberry polyploids in vitro. J Hered 73:377–378
Miyoshi K, Asakura N (1996) Callus induction, regeneration of haploid plants and chromosome doubling in ovule cultures of pot gerbera (Gerbera jamesonii). Plant Cell Rep 16:1–5
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nguyen T, Phuong T, Kenji U, Ikuo M, Yukio O, Hiroshi O (2003) Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell Tiss Organ Cult 72:19–25
Ochatt SJ, Patat-Ochatt EM, Moessner A (2011) Ploidy level determination within the context of in vitro breeding. Plant Cell Tiss Organ Cult 104:329–341
Orlikowska T, Nowak E, Marasek A, Kucharska D (1999) Effects of growth regulators and incubation period on in vitro regeneration of adventitious shoots from gerbera petiole. Plant Cell Tiss Organ Cult 59:95–102
Parthasarathy VA, Nagaraju V (1999) In vitro propagation in Gerbera jemesonii Bolus. Ind J Hortic 56:82–85
Pierik RLM, Steegmans HHM, Marelis JJ (1973) Gerbera plantlets from in vitro cultivated capitulum explants. Sci Hortic 1:117–119
Pinheiro AA, Pozzobon MT, do Valle CB, Penteado MIO, Carneiro VTC (2000) Duplication of the chromosome number of diploid Brachiaria brizantha plants using colchicine. Plant Cell Rep 19:274–278
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tiss Organ Cult 64:185–210
Rose JB, Kubba J, Tobutt KR (2001) Induction of tetraploids for breeding hardy ornamentals. Acta Hortic 560:109–112
Roy AT, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20:489–495
Shao JZ, Chen CL, Deng XX (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tiss Organ Cult 75:241–246
Shapiro HM (2003) Practical flow cytometry, 4th edn. John Wiley & Sons, Hoboken, N.J
Song P, Kang W, Peffley EB (1997) Chromosome doubling of Allium fistulosum 9 A. cepa interspecific F1 hybrids through colchicine treatment of regenerating callus. Euphytica 93:257–262
Speckman GJ, Post J, Dijkstra M (1965) The length of stomata as an indicator for polyploidy in rye-grass. Euphytica 14:225–230
Stebbins GL (1984) Polyploidy and the distributed of arctic-alpine flora: a new evidence and new approaches. Bot Helv 94:1–13
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tiss Organ Cult 102:213–220
Tosca A, Pandolfi R, Citterio S, Fasoli A, Sgorbati S (1995) Determination by flow cytometry of the chromosome doubling capacity of colchicine and oryzalin in gynogenetic haploids of Gerbera. Plant Cell Rep 14:455–458
Uhlik J (1981) Compendium postgradulans stadium for Genetics and Breeding. Prague V.S.Z. pp 105–195
Viehmannova I, Fernandez E, Bechyne M, Vyvadilova M, Greplova M (2009) In vitro induction of polyploidy in yacon (Smallanthus sonchifolius). Plant Cell Tiss Organ Cult 95:21–25
Wang L, Zheng SX, Gu ZJ (2002) In vitro culture of tetraploids of Aloe vera L. Acta Hortic Sinica 29:176–178
Wright JW (1976) Introduction of forest genetics. Academic Press, New York
Yang X, Ye CY, Cheng ZM, Tschaplinski TJ, Wullschleger SD, Yin W, Xia X, Tuskan GA (2011) Genomic aspects of research involving polyploid plants. Plant Cell Tiss Organ Cult 104:387–397
Zhang J, Zhang M, Deng X (2007) Obtaining autotetraplids in vitro at a high frequency in Citrus sinensis. Plant Cell Tiss Organ Cult 89:211–216
Zhang QY, Luo FX, Liu L, Guo FC (2010) In vitro induction of tetraploids in crape myrtle (Lagerstoemia indica L.). Plant Cell Tiss Organ Cult 101:41–47
Zhu ZT, Kang XY, Zhang ZY (1998) Studies on selection of natural triploids of Populus tomentosa. Sci Silvae Sinicae 34:22–31
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gantait, S., Mandal, N., Bhattacharyya, S. et al. Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tiss Organ Cult 106, 485–493 (2011). https://doi.org/10.1007/s11240-011-9947-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9947-1