Abstract
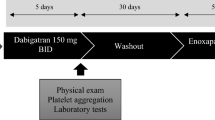
Heparin compounds, to include fractionated and unfractionated preparations, exert both antithrombotic and antiinflammatory effects through combined inhibition of factor Xa and thrombin. The contribution of modulated platelet activity in vivo is less clearly defined. The SYNERGY library was a prospectively designed repository for candidate clinical, hemostatic, platelet, and molecular biomarkers from patients participating in SYNERGY—a large-scale, randomized clinical trial evaluating the comparative benefits of unfractionated heparin (UFH) and enoxaparin in high-risk patients with acute coronary syndrome (ACS). Samples were collected from 201 patients enrolled at 26 experienced, participating sites and shipped to established core laboratories for analysis of platelet, endothelium-derived, inflammatory and coagulation activity biomarkers. Tissue factor pathway inhibitor (TFPI)—a vascular endothelial cell-derived factor Xa regulatory protein—correlated directly with plasma anti-Xa activity (unadjusted: r = 0.23, P < 0.0001; adjusted: β = 0.10; P = 0.001), as did TFPI–fXa complexes (unadjusted: r = 0.34, P < 0.0001; adjusted: β = 0.38; P = < 0.0001). In contrast, there was a direct and inverse relationship between anti-Xa activity and two platelet-derived biomarkers—plasminogen activator inhibitor-1 (unadjusted: r = −0.18, P = 0.0012; adjusted: β = −0.10; P = 0.021) and soluble CD40 ligand (unadjusted: r = −0.11, P = 0.05; adjusted: β = −0.13; P = 0.049). All measured analyte relationships persisted after adjustment for age, creatinine clearance, weight, sex, and duration of treatment. Differences in biomarkers between patients receiving UFH and those randomized to enoxaparin were not observed. The ability of heparin compounds to affect the prothrombotic and proinflammatory states which characterize ACS may involve factor Xa-related modulation of platelet activation and expression. Whether this potentially beneficial effect is direct or indirect and achieved, at least in part, through the release of endothelial cell-derived coagulation regulatory proteins will require further investigation.




Similar content being viewed by others
References
Koenig W, Khuseyinova N (2007) Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 27(1):15–26
Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105(9):1135–1143
Namba M et al (2007) Circulating platelet-derived microparticles are associated with atherothrombotic events: a marker for vulnerable blood. Arterioscler Thromb Vasc Biol 27(1):255–256
Waxman S, Ishibashi F, Muller JE (2006) Detection and treatment of vulnerable plaques and vulnerable patients: novel approaches to prevention of coronary events. Circulation 114(22):2390–2411
Petersen JL et al (2004) Coordinated series of studies to evaluate characteristics and mechanisms of acute coronary syndromes in high-risk patients randomly assigned to enoxaparin or unfractionated heparin: design and rationale of the SYNERGY Library. Am Heart J 148:269–276
Ferguson JJ et al (2004) Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. J Am Med Assoc 292(1):45–54
Falk E (2006) Pathogenesis of atherosclerosis. J Am Coll Cardiol 47(8 Suppl):C7–C12
Becker RC et al (1996) Relation between systemic anticoagulation as determined by activated partial thromboplastin time and heparin measurements and in-hospital clinical events in unstable angina and non-Q wave myocardiaL infarction. Thrombolysis in myocardial ischemia III B investigators. Am Heart J 131(3):421–433
Wagenvoord R et al (2008) Linear diffusion of thrombin and factor Xa along the heparin molecule explains the effects of extended heparin chain lengths. Thromb Res 122(2):237–245
Becker RC et al (2002) Influence of patient characteristics and renal function on factor Xa inhibition pharmacokinetics and pharmacodynamics after enoxaparin administration in non-ST-segment elevation acute coronary syndromes. Am Heart J 143:753–759
Montalescot G et al (2006) Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med 355(10):1006–1017
Montalescot G et al (2008) Impact of anticoagulation levels on outcomes in patients undergoing elective percutaneous coronary intervention: insights from the STEEPLE trial. Eur Heart J 29(4):462–471
Allen GA et al (2004) Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost 2(3):402–413
Kempton CL et al (2005) Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol 25(4):861–866
Cirino G et al (1997) Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Investig 99(10):2446–2451
Daubie V et al (2006) Factor Xa and thrombin evoke additive calcium and proinflammatory responses in endothelial cells subjected to coagulation. Biochim Biophys Acta 1763(8):860–869
Rapaport SI, Rao LV (1992) Initiation and regulation of tissue factor-dependent blood coagulation. Arterioscler Thromb 12(10):1111–1121
Broze GJ Jr (1992) The role of tissue factor pathway inhibitor in a revised coagulation cascade. Semin Hematol 29(3):159–169
Bajaj MS et al (1999) Transcriptional expression of tissue factor pathway inhibitor, thrombomodulin and von Willebrand factor in normal human tissues. Thromb Haemost 82(3):1047–1052
Golino P et al (2003) Involvement of tissue factor pathway inhibitor in the coronary circulation of patients with acute coronary syndromes. Circulation 108(23):2864–2869
Li Y et al (2002) Comparative effects of unfractionated heparin and low molecular weight heparin on vascular endothelial cell tissue factor pathway inhibitor release: a model for assessing intrinsic thromboresistance. J Thromb Thrombolysis 14(2):123–129
Becker RC et al (2004) Vascular endothelial tissue factor pathway inhibitor kinetics in culture following exposure to DX-9065a–a selective and direct factor Xa inhibitor. J Thromb Thrombolysis 18(3):193–197
Henn V et al (1998) CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391(6667):591–594
Michelson AD et al (2001) Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104(13):1533–1537
Furman MI et al (2001) Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol 38(4):1002–1006
Nakamura R et al (2006) Less pronounced enhancement of thrombin-dependent inactivation of plasminogen activator inhibitor type 1 by low molecular weight heparin compared with unfractionated heparin. Thromb Haemost 95(4):637–642
Bianchini EP, Pike RN, Le Bonniec BF (2004) The elusive role of the potential factor X cation-binding exosite-1 in substrate and inhibitor interactions. J Biol Chem 279(5):3671–3679
Becker RC et al (1994) The clinical use of flow cytometry for assessing platelet activation in acute coronary syndromes. TIMI-III thrombosis and anticoagulation group. Coron Artery Dis 5(4):339–345
Brogren H et al (2004) Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood 104(13):3943–3948
Montalescot G et al (2003) Comparison of effects on markers of blood cell activation of enoxaparin, dalteparin, and unfractionated heparin in patients with unstable angina pectoris or non-ST-segment elevation acute myocardial infarction (the ARMADA study). Am J Cardiol 91(8):925–930
Montalescot G et al (2000) Effects of various anticoagulant treatments on von Willebrand factor release in unstable angina. J Am Coll Cardiol 36(1):110–114
Jayachandran M (2005) Estrogenic regulation of tissue factor and tissue factor pathway inhibitor in platelets. Am J Physiol Heart Circ Physiol 289(5):H1908–H1916
Xiao Z, Theroux P (1998) Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation 97(3):251–256
Landolfi R et al (1994) Effects of unfractionated and low molecular weight heparins on platelet thromboxane biosynthesis “in vivo”. Thromb Haemost 72(6):942–946
Miletich JP, Jackson CM, Majerus PW (1977) Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci USA 74(9):4033–4036
Heiden D, Mielke CH Jr, Rodvien R Jr (1977) Impairment by heparin of primary haemostasis and platelet [14C]5-hydroxytryptamine release. Br J Haematol 36(3):427–436
Fernandez F et al (1986) Hemorrhagic doses of heparin and other glycosaminoglycans induce a platelet defect. Thromb Res 43(4):491–495
De Candia E, De Cristofaro R, Landolfi R (1999) Thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin. Circulation 99(25):3308–3314
Heeschen C et al (2003) Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 348(12):1104–1111
Konishi N, Hiroe K, Kawamura M (2010) Synergistic effect of a factor Xa inhibitor, TAK-442, and antiplatelet agents on whole blood coagulation and arterial thrombosis in rats. Thrombo Res 126(2):124–129
Samama MM et al (2010) Assessment of laboratory assays to measure rivaroxaban–an oral, direct factor Xa inhibitor. Thromb Haemost 103(4):815–825
Acknowledgments
The authors would like to thank Shaun G. Goodman, MD, Gregg W. Stone, MD, Alexandra J. Lansky, MD, Marc Cohen, MD, Robert M. Califf, MD, and James J. Ferguson, MD, the site principal investigators and coordinators who participated in the SYNERGY library and Amanda McMillan for her editorial assistance.
Funding
Funded by sanofi-aventis.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, R.C., Mahaffey, K.W., Yang, H. et al. Heparin-associated anti-Xa activity and platelet-derived prothrombotic and proinflammatory biomarkers in moderate to high-risk patients with acute coronary syndrome. J Thromb Thrombolysis 31, 146–153 (2011). https://doi.org/10.1007/s11239-010-0532-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-010-0532-y