Summary
Purpose
Because raised matrix metalloprotease (MMP) levels are associated with glioma invasion and angiogenesis, we tested the efficacy of marimastat (MT) an orally active drug that can reduce MMP levels, in patients with gliomas.
Patients and Methods
A total of 162 patients with intracranial glioblastoma multiforme or gliosarcomas who had undergone surgery and radiotherapy participated in this multicenter, double-blind, placebo-controlled, parallel group study conducted at 20 institutions. Seventy-nine patients (57 male, 22 female, median age 58 years) were randomized to receive placebo (PB), and 83 patients (51 male, 32 female, median age 57 years) were randomized to receive MT, 10 mg orally twice daily, until tumor progression.
Results
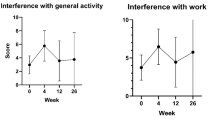
This intention-to-treat efficacy analysis showed no statistically significant difference between MT and PB groups with respect to survival (P=0.38, log rank test). The median survival time from protocol initiation was 37.9 weeks for the PB group and 42.9 weeks for the MT group, with a hazard ratio of 1.16 (95% CI 0.83 to 1.60). There were no statistically significant differences in quality of life between the PB and MT groups, as assessed by the FACT-BR questionnaire. Musculoskeletal toxicities led to dose modification or withdrawal in 20% of MT-treated and 1.2% of PB-treated patients.
Conclusion
MT does not improve survival in patients with glioblastoma or gliosarcoma following surgery and radiotherapy. Therefore, single-agent MT appears unwarranted; however, MT in combination with cytotoxic chemotherapy may be warranted, as suggested by observations in our study and other studies.
Similar content being viewed by others
References
Levin VA, Uhm JH, Jaeckle KA, et al, Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme Clin Cancer Res 6:3878–3884, 2000
Levin VA, Leibel SA, Gutin PH, Neoplasms of the central nervous system in DeVita VTJ, Hellman S, Rosenberg SA, (eds): Cancer: Principles and Practice of Oncology ed 6 Lippincott-Raven Philadelphia 2001, pp 2100–2160
Rao JS, Yamamoto M, Mohaman S, et al., Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP−9) in human gliomas Clin Exp Metastasis 14: 12–18, 1996
Sawaya RE, Yamamoto M, Gokaslan ZL, et al, Expression and localization of 72 kDa type IV collagenase (MMP−2) in human malignant gliomas in vivo Clin Exp Metastasis 14:35–42, 1996
Rao JS, Molecular mechanisms of glioma invasiveness: the role of proteases Nat Rev Cancer 3:489–501, 2003
Zucker S, Cao J, Chen WT, Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment Oncogene 19:6642–6650, 2000
Brown PD, Giavazzi R, Matrix metalloproteinase inhibition: a review of anti-tumour activity Ann Oncol 6:967–974, 1995
Nemunaitis J, Poole C, Primrose J, et al, Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies Clin Cancer Res 4:1101–1109, 1998
Burger PC, Vogel FS, Green SB, et al, Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications Cancer 56:1106–1111, 1985
Burger PC, Scheithauer BW, Vogel FS, Surgical Pathology of the Nervous System and its Coverings 3rd edn Churchill Livingstone New York 1991
Weitzner MA, Meyers CA, Byrne K, Psychosocial functioning and quality of life in patients with primary brain tumors J Neurosurg 84:29–34, 1996
Weitzner MA, Meyers CA, Gelke CK, et al, The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors Cancer 75:1151–1161, 1995
Macdonald DR, Cascino TL, Schold SC, Jr., et al, Response criteria for phase II studies of supratentorial malignant glioma J Clin Oncol 8:1277–1280, 1990
Levin VA, Crafts DC, Norman DM, et al, Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors J Neurosurg 47:329–335, 1977
Taves DR, Minimization: a new method of assigning patients to treatment and control groups Clin Pharmacol Ther 15:443–453, 1974
Leibel SA, Scott CB, Loeffler JS, Contemporary approaches to the treatment of malignant gliomas with radiation therapy Semin Oncol 21:198–219, 1994
Brown PD, Matrix metalloproteinase inhibitors: a novel class of anticancer agents Adv Enzyme Regul 35:293–301, 1995
Groves MD, Puduvalli VK, Hess KR, et al, Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme J Clin Oncol 20:1383–1388, 2002
Yung WK, Albright RE, Olson J, et al, A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse Br J Cancer 83:588–593, 2000
King J, Zhao J, Clingan P, et al, Randomised double blind placebo control study of adjuvant treatment with the metalloproteinase inhibitor, Marimastat in patients with inoperable colorectal hepatic metastases: significant survival advantage in patients with musculoskeletal side-effects Anticancer Res 23:639–645, 2003
Overall CM, Lopez-Otin C, Strategies for MMP inhibition in cancer: innovations for the post-trial era Nat Rev Cancer 2:657–672, 2002
Coussens LM, Fingleton B, Matrisian LM, Matrix metalloproteinase inhibitors and cancer: trials and tribulations Science 295:2387–2392, 2002
Patterson BC, Sang QA, Angiostatin-converting enzyme activities of human matrilysin (MMP−7) and gelatinase B/type IV collagenase (MMP−9) J Biol Chem 272:28823–28825, 1997
O’Reilly MS, Wiederschain D, Stetler-Stevenson WG, et al, Regulation of angiostatin production by matrix metalloproteinase−2 in a model of concomitant resistance J Biol Chem 274:29568–29571, 1999
Vazquez F, Hastings G, Ortega MA, et al, METH−1, a human ortholog of ADAMTS−1, and METH−2 are members of a new family of proteins with angio-inhibitory activity J Biol Chem 274:23349–23357, 1999
Toth M, Bernardo MM, Gervasi DC, et al, Tissue inhibitor of metalloproteinase (TIMP)−2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP−4 to enhance the (Membrane type 1)-MMP-dependent activation of pro-MMP−2 J Biol Chem 275:41415–41423, 2000
Osoba D, Brada M, Yung WKA, et al, Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme J Clin Oncol 18:1481–1491, 2000
Groves ES, Puduvalli V, Conrad CA, et al.: Temozolomide plus the matrix metalloproteinase inhibitor, marimastat, for recurrent anaplastic gliomas. J Clin Oncol submitted, 2006
Acknowledgement
We would like to thank Betty Notzon for editorial assistance and Melissa McLane for manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
★This work was presented in part at the 37th Annual Meeting of the American Society of Clinical Oncology, San Francisco, CA, on May 13, 2001, and supported by British Biotech Pharmaceuticals, Watlington Road, Oxford, OX4 6LY, UK. No author currently has a financial interest in Marimastat. Mark Baillet is currently with Origin Pharmaceutical Services Ltd.
*Participating institutions are listed in Table 1. Clinician authorship required ≥10 patient accrual from participating institution.
Rights and permissions
About this article
Cite this article
Levin, V.A., Phuphanich, S., Alfred Yung, W. et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation★ . J Neurooncol 78, 295–302 (2006). https://doi.org/10.1007/s11060-005-9098-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-005-9098-5