Abstract
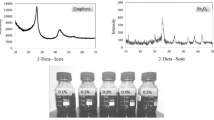
In the present study, the effect of volume concentration (0.05, 0.1 and 0.15 %) and temperature (10–90 °C) on viscosity and surface tension of graphene–water nanofluid has been experimentally measured. The sodium dodecyl benzene sulfonate is used as the surfactant for stable suspension of graphene. The results showed that the viscosity of graphene–water nanofluid increases with an increase in the volume concentration of nanoparticles and decreases with an increase in temperature. An average enhancement of 47.12 % in viscosity has been noted for 0.15 % volume concentration of graphene at 50 °C. The enhancement of the viscosity of the nanofluid at higher volume concentration is due to the higher shear rate. In contrast, the surface tension of the graphene–water nanofluid decreases with an increase in both volume concentration and temperature. A decrement of 18.7 % in surface tension has been noted for the same volume concentration and temperature. The surface tension reduction in nanofluid at higher volume concentrations is due to the adsorption of nanoparticles at the liquid–gas interface because of hydrophobic nature of graphene; and at higher temperatures, is due to the weakening of molecular attractions between fluid molecules and nanoparticles. The viscosity and surface tension showed stronger dependency on volume concentration than temperature. Based on the calculated effectiveness of graphene–water nanofluids, it is suggested that the graphene–water nanofluid is preferable as the better coolant for the real-time heat transfer applications.
















Similar content being viewed by others
Abbreviations
- C :
-
Coefficient of enhancement
- C p :
-
Specific heat/Jkg−1 K−1
- k :
-
Thermal conductivity/Wm−1 K−1
- T :
-
Temperature/°C
- \( \varphi \) :
-
Volume fraction
- \( \mu \) :
-
Dynamic viscosity/mN s m−2
- \( \sigma \) :
-
Surface tension/mN m−1
- \( \rho \) :
-
Density/kg m−3
- nf:
-
Nanofluid
- f:
-
Base fluid
- k:
-
Thermal conductivity
- μ :
-
Dynamic viscosity
- \( \infty \) :
-
Ambient
- P:
-
Nanoparticle
References
Selvakumar P, Suresh S. Convective performance of CuO/water nanofluid in an electronic heat sink. Exp Therm Fluid Sci. 2012;40:57–63.
Alfieri F, Tiwari MK, Zinovik I, Poulikakos D, Brunschwiler T, Michel B. 3D integrated water cooling of a composite multilayer stack of chips. J Heat Transf. 2010;132:121402.
Renfer A, Tiwari MK, Brunschwiler T, Michel B, Poulikakos D. Experimental investigation into vortex structure and pressure drop across microcavities in 3D integrated electronics. Exp Fluids. 2011;51:731–41.
Godson L, Lal DM, Wongwises S. Measurement of thermo physical properties of metallic nanofluids for high temperature applications. Nanoscale Microscale Thermophys Eng. 2010;14:152–73.
Godson L, Raja B, Lal DM, Wongwises S. Convective heat transfer characteristics of silver-water nanofluid under laminar and turbulent flow conditions. J Therm Sci Eng Appl. 2012;4(3):1–8.
Asirvatham LG, Raja B, Lal DM, Wongwises S. Convective heat transfer of nanofluids with correlations. Particuology. 2011;9:626–31.
Wong KV, Leon OD. Applications of nanofluids: current and future. Adv Mech Eng. 2010;2010:1–11.
Mahian O, Kianifar A, Kalogirou SA, Pop I, Wongwises S. A review of the applications of nanofluids in solar energy. Int J Heat Mass Transf. 2013;57:582–94.
Mahbubul IM, Saidur R, Amalina MA. Latest developments on the viscosity of nanofluids. Int J Heat Mass Transf. 2012;55:874–85.
Saidur R, Leong KY, Mohammad HA. A review on applications and challenges of nanofluids. Renew Sustain Energy Rev. 2011;15:1646–68.
Godson L, Raja B, Lal DM, Wongwises S. Enhancement of heat transfer using nanofluids—an overview. Renew Sustain Energy Rev. 2010;14:629–41.
Thomas S, Sobhan CBP. A review of experimental investigations on thermal phenomena in nanofluids. Nanoscale Res Lett. 2011;6:377.
Shanthi R, Sundaram SA, Velraj R. Heat transfer enhancement using nanofluids—an overview. Therm Sci. 2012;16:423–44.
Yiamsawas T, Dalkilic AS, Mahian O, Wongwises S. Measurement and correlation of the viscosity of water-based Al2O3 and TiO2 nanofluids in high temperatures and comparisons with literature reports. J Dispers Sci Technol. 2013;34(12):1697–703.
Aladag B, Salma H, Doner N, Mare T, Steven D, Estelle P. Experimental investigations of the viscosity of nanofluids at low temperatures. Appl Energy. 2012;97:876–80.
Hosseini SM, Moghadassi AR, Henneke DE. A new dimensionless group model for determining the viscosity of nanofluids. J Therm Anal Calorim. 2010;100:873–7.
Hemmat EM, Saedodin S, Wongwises S, Toghraie D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluid. J Therm Anal Calorim. 2015;119:1817–24.
Silambarasan M, Manikandan S, Rajan KS. Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int J Heat Mass Transf. 2012;55:7991–8002.
Fedele L, Colla L, Bobbo S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int J Refrig. 2012;35:1359–66.
Tao W, Jiang NM, Yang LZ, Hui SC, KeFa C. Viscosity and aggregation structure of nanocolloidal dispersions. Chin Sci Bull. 2012;57:3644–51.
Duan F, Kwek D, Crivoi A. Viscosity affected by nanoparticle aggregation in Al2O3-water nanofluids. Nanoscale Res Lett. 2011;6:248.
Rudyak VY, Dimov SV, Kuznetsov VV. On the dependence of the viscosity coefficient of nanofluids on particle size and temperature. Tech Phys Lett. 2013;39:779–82.
Kole M, Dey TK. Investigation of thermal conductivity, viscosity, and electrical conductivity of graphene based nanofluids. J Appl Phys. 2013;113:084307.
Yang JC, Li FC, Zhou WW, He YR, Jiang BC. Experimental investigation on the thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids. Int J Heat Mass Transf. 2012;55:3160–6.
Utomo AT, Poth H, Robbins PT, Pacek AW. Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int J Heat Mass Transf. 2012;55:7772–81.
Kole M, Dey TK. Effect of aggregation on the viscosity of copper oxide-gear oil nanofluids. Int J Therm Sci. 2011;50:1741–7.
Dehkordi BL, Kazi SN, Hamdi M, Ghadimi A, Sadeghinezhad E, Metselaar HSC. Investigation of viscosity and thermal conductivity of alumina nanofluids with addition of SDBS. Heat Mass Transf. 2013;49:1109–15.
Vasheghani M, Marzbanrad E, Zamani C, Aminy M, Raissi B, Ebadzadeh T, Bafrooei HB. Effect of Al2O3 phases on the enhancement of thermal conductivity and viscosity of nanofluids in engine oil. Heat Mass Transf. 2011;47:1401–5.
Zhu DS, Wu SY, Wang N. Surface tension and viscosity of aluminum oxide nanofluids. In: Proceedings of 6th international symposium on multiphase flow, heat mass transfer and energy conversion, vol. 460, July 11–15, Xi’an, China; 2009.
Wang F, Han L, Zhang Z, Fang X, Shi J, Ma W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res Lett. 2012;73:14.
Tiwari AK, Ghosh P, Sarkar J. Heat transfer and pressure drop characteristics of CeO2/water nanofluid in plate heat exchanger. Appl Therm Eng. 2013;57:24–32.
Gallego MJP, Casanova C, Legido JL, Piñeiro MM. CuO in water nanofluid: influence of particle size and polydispersity on volumetric behaviour and viscosity. Fluid Phase Equilib. 2011;300(1–2):188–96.
Rashin MN, Hemalatha J. Viscosity studies on novel copper oxide–coconut oil nanofluid. Exp Therm Fluid Sci. 2013;48:67–72.
Mehrali M, Sadeghinezhad E, Latibari ST, Mehrali M, Togun H, Zubir MNM, Kazi SN, Metselaar HSC. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res Lett. 2014;9(15):1–12.
Moosavi M, Goharshadi EK, Youssefi A. Fabrication, characterization, and measurement of some physicochemical properties of ZnO nanofluids. Int J Heat Fluid Flow. 2010;31(4):599–605.
Khaleduzzaman SS, Mahbubul IM, Shahrul IM, Saidur R. Effect of particle concentration, temperature and surfactant on surface tension of nanofluids. Int Comm Heat Mass Transf. 2013;49:110–4.
Godson L, Raja B, Raj V, Lal DM. Measurement of viscosity and surface tension of silver deionized water nanofluids. In: Proceedings of 37th national & 4th international conference on fluid mechanics and fluid power, vol. no. 2010, December 16–18, IIT Madras, Chennai, India; 2010.
Godson L, Deepak K, Enoch C, Jefferson B, Raja B. Heat transfer characteristics of silver/water nanofluids in a shell and tube heat exchanger. Arch Civil Mech Eng. 2014;14:489–96.
Asirvatham LG, Nimmagadda R, Wongwises S. Heat transfer performance of screen mesh wick heat pipes using silver-water nanofluid. Int J Heat Mass Transf. 2013;60:201–9.
Asirvatham LG, Nimmagadda R, Wongwises S. Operational limitations of heat pipes with silver-water nanofluids. J Heat Transf. 2013;135:1–10.
Kumaresan G, Venkatachalapathy S, Asirvatham LG, Wongwises S. Comparative study on heat transfer characteristics of sintered and mesh wick heat pipes using CuO nanofluids. Int Comm Heat Mass Transf. 2014;57:208–15.
Incropera FP, DeWeitt DP. Fundamentals of heat and mass transfer. 5th ed. New York: Wiley; 2005.
Prasher R, Song D, Wang J. Measurements of nanofluid viscosity and its implications for thermal applications. Appl Phys Lett. 2006;89:1–3.
Brinkman H. The viscosity of concentrated suspensions and solutions. J Chem Phys. 1952;20:571.
Wang X, Xu X, Choi SUS. Thermal conductivity of nanoparticle–fluid mixture. J Thermophys Heat Transf. 1999;13(4):474–80.
Buongiorno J. Convective transport in nanofluids. J Heat Transf. 2006;128:240–50.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the funding agency, the Department of Science and Technology (DST), Science and Engineering Research Board (SERB), (SB/FTP/ETA-362/2012), New Delhi, India. The third author would like to thank the National Science and Technology Development Agency for the support, the “Research Chair Grant” National Science and Technology Development Agency (NSTDA), the Thailand Research Fund (IRG5780005) and the National Research University Project (NRU) for the support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahammed, N., Asirvatham, L.G. & Wongwises, S. Effect of volume concentration and temperature on viscosity and surface tension of graphene–water nanofluid for heat transfer applications. J Therm Anal Calorim 123, 1399–1409 (2016). https://doi.org/10.1007/s10973-015-5034-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5034-x