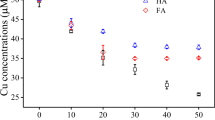
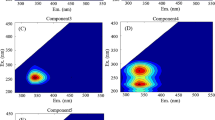
Abstract
Significant quenching of fluorescence by Cu in rainwater samples from southeastern North Carolina demonstrates that chromophoric dissolved organic matter (CDOM) is an effective ligand for Cu in rainwater. A strong inverse correlation between the decrease in fluorescence upon Cu addition and CDOM abundance suggests the presence of excess binding sites for Cu in high CDOM samples. Electroanalytical studies indicate that CDOM extracted from C18 cartridges formed Cu complexes with concentrations and conditional stability constants similar to ligands found in ambient rainwater. When authentic rainwater samples were photolyzed with simulated sunlight both photoproduction and photodestruction of ligands were observed, suggesting the photochemical response of Cu-complexing ligands in rainwater is the result of two competing reactions. The rate of CDOM photobleaching was directly related to changes in strong ligands (KCuL ∼ 1015) whereas weaker ligands (KCuL < 1013) were not correlated, suggesting the photolabile CDOM resides in the strong ligand class. A photolysis study comparing filtered and unfiltered rainwater samples indicated that Cu-complexing ligands adsorbed onto or otherwise associated with particles are photodegraded much more rapidly than dissolved ligands. Photolysis with UV radiation appears to be most effective at engendering changes in Cu ligands, however a significant photochemical response was also observed when samples were exposed to photosynthetically active radiation with wavelengths greater than 400 nm. Results from this study demonstrate that complexation of Cu by CDOM has important ramifications for controlling both the speciation of the metal and the reactivity of CDOM in rainwater.







Similar content being viewed by others
References
Arimoto, R., Duce, R.A., Ray, B.J., Unni, C.K.: Atmospheric trace-elements at Enewetak Atoll: 2. Transport to the ocean by wet and dry deposition. J. Geophys. Res.-Atmos. 90, 2391–2408 (1985)
Astrom, M., Corin, N.: Abundance, sources and speciation of trace elements in humus-rich streams affected by acid sulphate soils. Aquat. Geochem. 6, 367–383 (2000)
Berger, P., Ewald, M., Liu, D., Weber, J.H.: Application of the florescence quenching titration method to the complexation of copper(II) in the Gironde estuary (France). Mar. Chem. 14, 289–295 (1984)
Boehme, J., Coble, P., Conmy, R., Stovall-Leonard, A.: Examining CDOM fluorescence variability using principal component analysis: seasonal and regional modeling of three-dimensional fluorescence in the Gulf of Mexico. Mar. Chem. 89, 3–14 (2004)
Boussemart, M., Benamou, C., Richou, M., Benaim, J.Y.: Comparison of differential pulse anodic-stripping voltammetry and spectrofluorometry for determination of complexes between copper and organic-matter in interstitial waters extracted from marine-sediments. Mar. Chem. 28, 27–39 (1989)
Brinkmann, T., Sartorius, D., Frimmel, F.H.: Photobleaching of humic rich dissolved organic matter. Aquat. Sci. 65, 415–424 (2003)
Bruland, K.W., Rue, E.L., Donat, J.R., Skrabal, S.A., Moffett, J.W.: Intercomparison of voltammetric techniques to determine the chemical speciation of dissolved copper in a coastal seawater sample. Anal. Chim. Acta 405, 99–113 (2000)
Campos, M.L.A.M., Van den Berg, C.M.G.: Determination of copper complexation in sea water by cathodic stripping voltammetry and ligand competition with salicyclaldoxime. Anal. Chim. Acta 284, 481–496 (1994)
Chen, X.F., Cheng, P., Liu, X., Zhao, B., Liao, D.Z., Yan, S.P., Jiang, Z.H.: Two-dimensional coordination polymers of copper(II) with oxalate: lattice water control of structure. Inorg. Chem. 40 2652–2659 (2001)
Church, T.M., Tramontano, J.M., Scudlark, J.R., Jickells, T.D., Tokos, J.J., Knap, A.H., Galloway, J.N.: The wet deposition of trace-metals to the western Atlantic ocean at the mid-Atlantic coast and on Bermuda. Atmos. Environ. 18, 2657–2664 (1984)
Coble, P.G.: Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar. Chem. 51, 325–346 (1996)
Coble, P.G., Schultz, C.A., Mopper, K.: Fluorescence contouring analysis of DOC intercalibration experiment samples – a comparison of techniques. Mar. Chem. 41, 173–178 (1993)
Coble, P.G., Del Castillo, C.E., Avril, B.: Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep-Sea Res., Part 2, Top. Stud. Oceanogr. 45, 2195–2223 (1998)
Croot, P.L., Moffett, J.W., Luther, G.W.: Polarographic determination of half-wave potentials for copper–organic complexes in seawater. Mar. Chem. 67, 219–232 (1999)
Del Castillo, C.E., Coble, P.G., Morell, J.M., Lopez, J.M., Corredor, J.E.: Analysis of the optical properties of the Orinoco River plume by absorption and fluorescence spectroscopy. Mar. Chem. 66, 35–51 (1999)
Draxler, R.R., Rolph, G.D.: HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) model access via NOAA ARL READY. NOAA Air Resources Laboratory, Silver Spring, MD. http://www.arl.noaa.gov/ready/hysplit4.html) (2003)
DuPont: Information bulletin: high performance films. DuPont FEP fluorocarbon film. Reorder no.: H-55007-2. http://www2.dupont.com/Teflon_Industrial/en_US/assets/downloads/h55007.pdf (1996)
Ferraudi, G., Muralidharan, S.: Photochemical properties of copper complexes. Coord. Chem. Rev. 36, 45–88 (1981)
Graber, E.R., Rudich, Y.: Atmospheric HULIS: How humic-like are they? A comprehensive and critical review. Atmos. Chem. Phys. 6, 729–753 (2006)
Graedel, T.E., Weschler, C.J., Mandich, M.L.: Influence of transition metal complexes on atmospheric droplet acidity. Nature 317, 240–242 (1985)
Graedel, T.E., Mandich, M.L., Weschler, C.J.: Kinetic model studies of atmospheric droplet chemistry 2. Homogeneous transition metal chemistry in raindrops. J. Geophys. Res. 91, 5205–5221 (1986)
Herrmann, H., Ervens, B.H.-W., Jacobi, H., Wolke, R., Nowacki, P., Zellner R.: CAPRAM2.3: A chemical aqueous phase radical mechanism for tropospheric chemistry. J. Atmos. Chem. 36, 231–284. (2000)
Kerminen, V.-M., Ojanen, C., Pakkanen, T., Hillamo, R., Aurela, M., Merilainen, J.: Low-molecular-weight dicarboxylic acids in an urban and rural atmosphere. J. Aerosol Sci. 31, 349–362 (2000)
Khwaja, H.A.: Atmospheric concentrations of carboxylic-acids and related-compounds at a semiurban site. Atmos. Environ. 29, 127–139 (1995)
Kieber, R.J., Williams, K., Willey, J.D., Skrabal, S., Avery, G.B.: Iron speciation in coastal rainwater: concentration and deposition to seawater. Mar. Chem. 73, 83–95 (2001)
Kieber, R.J., Skrabal, S.A., Smith, C., Willey, J.D.: Redox speciation of copper in rainwater: temporal variability and atmospheric deposition. Environ. Sci. Technol. 38, 3587–3594 (2004)
Kieber, R.J., Skrabal, S.A., Smith, B.J., Willey, J.D.: Organic complexation of Fe(II) and its impact on the redox cycling of iron in rain. Environ. Sci. Technol. 39, 1576–1583 (2005)
Kieber, R.J., Whitehead, R.F., Reid, S.N., Willey, J.D., Seaton, P.J.: Chromophoric dissolved organic matter (CDOM) in rainwater, south-eastern North Carolina, USA. J. Atmos. Chem. 54, 21–41 (2006)
Krivacsy, Z., Kiss, G., Varga, B., Galambos, I., Sarvari, Z., Gelencser, A., Molnar, A., Fuzzi, S., Facchini, M.C., Zappoli, S., Andracchio, A., Alsberg, T., Hansson, H.C., Persson, L.: Study of humic-like substances in fog and interstitial aerosol by size-exclusion chromatography and capillary electrophoresis. Atmos. Environ. 34, 4273–4281 (2000)
Laglera, L.M., van den Berg, C.M.G.: Photochemical oxidation of thiols and copper complexing ligands in estuarine waters. Mar. Chem. 101, 130–140 (2006)
Leal, M.F.C., Van den Berg, C.M.G.: Evidence for strong copper(I) complexation by organic ligands in seawater. Aquat. Geochem. 4, 49–75 (1998)
Likens, G.E., Edgerton, E.S., Galloway, J.N.: The composition and deposition of organic carbon in precipitation. Tellus 35B, 16–24 (1983)
Losno, R.: Trace metals acting as catalysts in a marine cloud: a box model study. Phys. Chem. Earth B 24, 281–286 (1999)
Madronich S.: Photodissociation in the atmosphere .1. Actinic flux and the effects of ground reflections and clouds. J. Geophys. Res.-Atmos. 92, 9740–9752 (1987)
Matthijsen, J., Builtjes, P.J.H., Sedlak, D.L.: Cloud model experiments of the effect of iron and copper on tropospheric ozone under marine and continental conditions. Meteorol. Atmos. Phys. 57, 43–60 (1995)
Midorikawa, T., Tanoue, E.: Molecular masses and chromophoric properties of dissolved organic ligands for copper(II) in oceanic water. Mar. Chem. 62, 219–239 (1998)
Mukai, H., Ambe, Y.: Characterization of humic acid-like brown substance in airborne particulate matter and tentative identification of its origin. Atmos. Environ. 20, 813–819 (1986)
Nimmo, M., Fones, G.R.: The potential pool of Co, Ni, Cu, Pb and Cd organic complexing ligands in coastal and urban rain waters. Atmos. Environ. 31, 693–702 (1997)
Okochi, H., Brimblecombe, P.: Potential Trace Metal-Organic Complexation in the Atmosphere. Sci. World J. 2, 767–786 (2002)
Plaza, C., D’Orazio, V., Senesi, N.: Copper(II) complexation of humic acids from the first generation of EUROSOILS by total luminescence spectroscopy. Geoderma 125, 177–186 (2005)
Ryan, D.K., Weber, J.H.: Copper(II) complexing capacities of natural waters by fluorescence quenching. Environ. Sci. Technol. 16, 866–872 (1982)
Sander, S., Kim, J.P., Anderson, B., Hunter, K.A.: Effect of UVB irradiation on Cu2+ binding organic ligands and Cu2+ speciation in alpine lake waters of New Zealand. Environ. Chem. 2, 56–62. (2005)
Scatchard, G.: The attractions of proteins for small molecules and ions. Ann. N.Y. Acad. Sci. 51, 660–672 (1949)
Sedlak, D.L., Hoigné, J.: The role of copper and oxalate in the redox cycling of iron in atmospheric waters. Atmos. Environ. 27A, 2173–2185 (1993)
Seitzinger, S.P., Styles, R.M., Lauck, R., Mazurek, M.A.: Atmospheric pressure mass spectrometry: a new analytical chemical characterization method for dissolved organic matter in rainwater. Environ. Sci. Technol. 37, 131–137 (2003)
Shank, G.C., Skrabal, S.A., Whitehead, R.F., Kieber, R.J.: Strong copper complexation in an organic-rich estuary: the importance of allochthonous dissolved organic matter. Mar. Chem. 88, 21–39 (2004)
Shank, G.C., Whitehead, R.F., Smith, M.L., Skrabal, S.A., Kieber, R.J.: Photodegradation of strong copper-complexing ligands in organic-rich estuarine waters. Limnol. Oceanogr. 51, 884–892 (2006)
Spokes, L.J., Campos, M.L.A.M., Jickells, T.D.: The role of organic matter in controlling copper speciation in precipitation. Atmos. Environ. 30, 3959–3966 (1996)
Stedmon, C.A., Markager, S.: Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchments using PARAFAC analysis. Limnol. Oceanogr. 50, 686–697 (2005)
Stevenson, F.J.: Humus chemistry. Genesis, composition, reactions., John Wiley and Sons. (1982)
Sunda, W., Guillard, R.R.L.: The relationship between cupric ion activity and the toxicity to phytoplankton. J. Mar. Res. 34, 511–529 (1976)
Tramontano, J.M., Scudlark, J.R., Church, T.M.: A method for the collection, handling and analysis of trace metals in precipitation. Environ. Sci. Technol. 21, 749–753 (1987)
Van Leeuwen, H.P., Town, R.M.: Kinetic limitations in measuring stabilities of metal complexes by competitive ligand exchange-adsorptive stripping voltammetry (CLE-AdSV). Environ. Sci. Technol. 39, 7217–7225 (2005)
Ventry, L.S., Ryan, D.K., Gilbert, T.R.: A rapid fluorescence quenching method for the determination of equilibrium parameters for copper(II) complexation by humic materials. Microchem. J. 44, 201–214 (1991)
Voelker, B.M., Sedlak, D.L., Zafiriou, O.C.: Chemistry of superoxide radical in seawater: reactions with organic Cu complexes. Environ. Sci. Technol. 34, 1036–1042 (2000)
Wang, C., Zhu, B., Li, H.: Theoretical analysis and determination of the heterogeneous stability constant of copper(II)-humic acids complex at chemically modified carbon paste electrode. Electroanalysis 11, 183–187 (1999)
Warneck, P.: In-cloud chemistry opens pathway to the formation of oxalic acid in the marine atmosphere. Atmos. Environ., A Gen. Topics 37, 2423–2427 (2003)
Wayne, R.P.: Chemistry of Atmospheres (2nd ed). Oxford University Press, Oxford (1999)
Weschler, C.J., Mandich, M.L., Graedel, T.E.: Speciation, photosensitivity, and reactions of transition metal ions in atmospheric droplets. J. Geophys. Res. 91, 5189–5204 (1986)
Willey, J.D.: The effect of seawater magnesium on natural fluorescence during estuarine mixing, and implications for tracer applications. Mar. Chem. 15, 19–45 (1984)
Willey, J.D., Kieber, R.J., Eyman, M.S., Avery, G.B., Jr.: Rainwater dissolved organic carbon: concentrations and global flux. Glob. Biogeochem. Cycles 14, 139–148 (2000)
Witt, M., Jickells, T.D.: Copper complexation in marine and terrestrial rain water. Atmos. Environ. 37, 7657–7666 (2005)
Witt, M., Skrabal, S., Kieber, R., Willey, J.: Copper complexation in coastal rainwater, southeastern USA. Atmos. Environ. 41, 3619–3630 (2007) DOI 3610.1016/j.atmosenv.2006.3612.3038
Wu, C.-H., Sun, L., Faust, B.C.: Photochemical formation of copper(I) from copper(II)-dicarboxylate complexes: effects of outer-sphere versus inner-sphere coordination and of quenching by malonate. J. Phys. Chem. A 104, 4989–4996 (2000)
Yokoi, K., Tomisaki, T., Koide, T., Vandenberg, C.M.G.: Effective UV photolytic decomposition of organic-compounds with a low-pressure mercury lamp as pre-treatment for voltammetric analysis of trace-metals. Fresenius J. Anal. Chem. 352, 547–549 (1995)
Acknowledgments
This research was supported by the National Science Foundation through grants ATM-0342420 and OCE-0326685. The Marine and Atmospheric Chemistry Research Laboratory group at UNCW assisted with sampling and analyses. The authors thank the maintenance staff at UNCW for their assistance with the running of the rain collection site. We are grateful to 2 anonymous reviewers for their thoughtful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witt, M.L.I., Skrabal, S., Kieber, R. et al. Photochemistry of Cu complexed with chromophoric dissolved organic matter: implications for Cu speciation in rainwater. J Atmos Chem 58, 89–109 (2007). https://doi.org/10.1007/s10874-007-9079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-007-9079-5