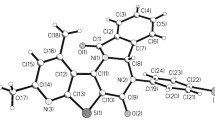
N-Acyl cytisine derivatives were synthesized by acylation with acetic anhydride; benzoyl and o-bromo- and p-nitrobenzoyl chlorides; and crotonyl and cinnamoyl chlorides. The structures of the synthesized compounds were studied using IR, PMR, and x-ray structure analysis (XSA). PMR spectra of the N-acylcytisines in solution typically had two rotational isomers around the N12–CO bond. Conformational analysis was performed using XSA for the position of the acyl group relative to the cytisine core. Bond lengths and angles of the acyl groups involved in the conjugation were analyzed.



Similar content being viewed by others
References
A. S. Sadykov, Kh. A. Aslanov, and Yu. K. Kushmuradov, Quinolizidine Alkaloids [in Russian], Nauka, Moscow, 1975.
M. D. Mashkovsii, Drugs [in Russian], Vol. 1, Meditsina, Moscow, 1998.
M. K. Ibraev, D. M. Turdybekov, S. D. Fazylov, K. M. Turdybekov, A. M. Gazaliev, and T. S. Zhivotova, Zh. Org. Khim., 40, 752 (2004).
B. A. Salakhutdinov, D. N. Dalimov, T. F. Aripov, I. I. Tukfatulina, R. Kh. Ziyatdinova, A. Zh. Zhuraev, F. G. Kamaev, L. Yu. Izotova, B. T. Ibragimov, I. Mavridis, and P. Guastas, Khim. Prir. Soedin., 209 (2002).
T. V. Khakimova, O. A. Pukhlyakova, G. A. Shovaleeva, A. A. Fatykhov, E. V. Vasil′eva, and L. V. Spirikhin,Khim. Prir. Soedin., 301 (2001).
K. A. Krasnov, V. G. Kartsev, A. S. Gorovoi, and V. N. Khrustalev, Khim. Prir. Soedin., 367 (2002).
R. Tlegenov, Synthesis, Structure, and Properties of Derivatives of Lupinine, Anabasine, Cytisine, and Several N-containing Heterocycles (Thesis), Institute of Bioorganic Chemistry, Academy of Sciences of the Republic of Uzbekistan, Tashkent, 2008.
Z. I. Primukhamedov and K. S. Tillyaev, Uzb. Khim. Zh., No. 1, 52 (1981).
Z. I. Primukhamedov, K. S. Tillyaev, and R. A. Zaidova, Uzb. Khim. Zh., No. 3, 63 (1982).
Z. I. Primukhamedov and K. S. Tillyaev, Uzb. Khim. Zh., No. 3, 22 (1978).
V. A. Saprykina, V. I. Vinogradova, R. F. Ambartsumova, T. F. Ibragimov, and Kh. M. Shakhidoyatov, Khim. Prir. Soedin., 379 (2006).
V. A. Saprykina, V. I. Vinogradova, R. F. Ambartsumova, T. F. Ibragimov, A. Sultankulova, and Kh. M. Shakhidoyatov, Khim. Prir. Soedin., 479 (2004).
Kh. U. Usmanov, R. S. Tillyaev, U. N. Musaev, and M. M. Mirkhidoyatov, Dokl. Akad. Nauk UzSSR, No. 12, 22 (1970).
Acknowledgment
The work was financed by the KKRNT RU program for Basic Scientific Research, Grant FA-F3-T045.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 702–707, November–December, 2009.
Rights and permissions
About this article
Cite this article
Abdullaev, N.P., Makhmudov, U.S., Tashkhodzhaev, B. et al. Structural features of N-acylcytisines. Chem Nat Compd 45, 837–843 (2009). https://doi.org/10.1007/s10600-010-9495-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-010-9495-7