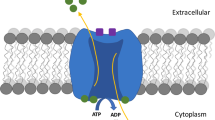
Abstract
Extracellular/intracellular l-[14C]glutamate exchange and conservativeness of the extracellular level of l-[14C]glutamate was analyzed in isolated rat brain nerve terminals. l-Glutamate-, dl-threo-β-hydroxyaspartate (dl-THA)-, and d-aspartate-induced increase in the ambient level of l-[14C]glutamate or d-[3H]aspartate was evaluated comparatively. 100 μM “cold” nonradiolabeled l-glutamate, dl-THA, d-aspartate extruded a quarter of radioactivity from l-[14C]glutamate-preloaded synaptosomes for 6 min. The similar results were obtained with l-glutamate-evoked extracellular/intracellular redistribution of d-[3H]aspartate. Contribution of presynaptic glutamate receptors to an increase in the extracellular l-[14C]glutamate level was evaluated using receptor agonists NMDA, AMPA, and kainate (100 μM), and it consisted of less than 5 % of total accumulated label. The existence of the efficient extracellular/intracellular glutamate exchange, and so dynamic glutamate gradient across the plasma membrane of nerve terminals was demonstrated. A two-substrate kinetic algorithm that included transporter reversal was considered. The extracellular level of l-[14C]glutamate and d-[3H]aspartate in nerve terminals depended on the amount of exogenous substrates of glutamate transporter available. Taking into account that l-glutamate, dl-THA, and d-aspartate are the substrates of glutamate transporters, and also the similarity in their effectiveness in the establishment of new extracellular level of the neurotransmitters, the central role of glutamate transporters in permanent glutamate turnover in nerve terminals was demonstrated.






Similar content being viewed by others
References
Borisova T (2013) Cholesterol and presynaptic glutamate transport in the brain. Springer, New York
Borisova T (2016) Permanent dynamic transporter-mediated turnover of glutamate across the plasma membrane of presynaptic nerve terminals: arguments for and against. Rev Neurosci 27(1):71–81
Borisova T, Borysov A (2016) Putative duality of presynaptic events. Rev Neurosci. doi:10.1515/revneuro-2015-0044
Borisova T, Himmelreich N (2005) Centrifuge-induced hypergravity: [3H]GABA and l-[14C]glutamate uptake, exocytosis and efflux mediated by high-affinity, sodium-dependent transporters. Adv Space Res 36:1340–1345
Borisova T, Krisanova N (2008) Presynaptic transporter-mediated release of glutamate evoked by the protonophore FCCP increases under altered gravity conditions. Adv Space Res 42:1971–1979
Borisova T, Krisanova N, Himmelreich N (2004) Exposure of animals to artificial gravity conditions leads to the alteration of the glutamate release from rat cerebral hemispheres nerve terminals. Adv Space Res 33:1362–1367
Borisova T, Krisanova N, Sivko R, Borysov A (2010a) Cholesterol depletion attenuates tonic release but increases the ambient level of glutamate in rat brain synaptosomes. Neurochem Int 56:466–478
Borisova T, Sivko R, Borysov A, Krisanova N (2010b) Diverse presynaptic mechanisms underlying methyl-beta-cyclodextrin – mediated changes in glutamate transport. Cell Mol Neurobiol 30(7):1013–1023
Borysov A, Krisanova N, Chunihin O, Ostapchenko L, Pozdnyakova N, Borisova T (2014) A comparative study of neurotoxic potential of synthesized polysaccharide-coated and native ferritin-based magnetic nanoparticles. Croat Med J 55:195–205
Cavelier P, Attwell D (2005) Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol 564:397–410
Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D (2005) Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol 87:3–16
Chefer VI, Denoroy L, Zapata A, Shippenberg TS (2009) Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci 30:272–278
Cotman CW (1974) Isolation of synaptosomal and synaptic plasma membrane fractions. Methods Enzymol 31:445–452
Dalby NO, Mody I (2003) Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol 90:786–797
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA (2006) Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J Neurochem 96:1626–1635
Dzubay JA, Jahr CE (1999) The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci 19(13):5265–5274
Featherstone DE, Shippy SA (2008) Regulation of synaptic transmission by ambient extracellular glutamate. The Neurosci 14:171–181
Funicello M, Conti P, Amici MD, Micheli CD, Mennini T, Gobbi M (2004) Dissociation of [3H]l-glutamate uptake from l-glutamate-induced [3H]d-aspartate release by 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-4-carboxylic acid and 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-6-carboxylic acid, two conformationally constrained aspartate and glutamate analogs. Mol Pharmacol 66(3):522–529
González MI, Kazanietz MG, Robinson MB (2002) Regulation of the neuronal glutamate transporter excitatory amino acid carrier-1 (EAAC1) by different protein kinase C subtypes. Mol Pharmacol 62:901–910
Grewer C, Gameiro A, Zhang Z et al (2008) Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB Life 60(9):609–619
Herman MA, Jahr CE (2007) Extracellular glutamate concentration in hippocampal slice. J Neurosci 27:9736–9741
Jabaudon D, Shimamoto K, Yasuda-Kamatani Y (1999) Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc. Natl. Acad. Sci. USA 96:8733–8738
Janáky R, Dohovics R, Hermann A, Oja SS, Saransaari P (2001) Effects of metabotropic glutamate receptor agonists and antagonists on d-aspartate release from mouse cerebral cortical and striatal slices. Neurochem Res 26(11):1217–1224
Kanai Y, Hediger MA (2004) The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflug Arch 447:469–479
Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA (2013) The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med 34:108–120
Krisanova N, Trikash I, Borisova T (2009) Synaptopathy under conditions of altered gravity: changes in synaptic vesicle fusion and glutamate release. Neurochem Int 55:724–731
Larson E, Howlett B, Jagendorf A (1986) Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem 155:243–248
LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR (1995) GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15:1287–1298
Makarov V, Kucheryavykh L, Kucheryavykh Y, Rivera A, Eaton MJ, Skatchkov SN, Inyushin M (2013) Transport reversal during heteroexchange: a kinetic study. J Biophys. Article ID 683256
Manent JB, Represa A (2007) Neurotransmitters and brain maturation: early paracrine actions of GABA and glutamate modulate neuronal migration. Neuroscientist 13:268–279
McBean GJ (1994) Inhibition of the glutamate transporter and glial enzymes in rat striatum by the gliotoxin, alpha aminoadipate. Br J Pharmacol 113(2):536–540
Moussawi K, Riegel A, Nair S, Kalivas PW (2011) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5:94
Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G (2001) Neurotransmitters as early signals for central nervoussystemdevelopment. Cell Tissue Res 305:187–202
Nyitrai G, Kekesi KA, Juhasz G (2006) Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr Top Med Chem 6:935–940
Pendyam S, Mohan A, Kalivas PW, Nair SS (2012) Role of perisynaptic parameters in neurotransmitter homeostasis—computational study of a general synapse. Synapse 66:608–621
Pozdnyakova N, Dudarenko M, Yatsenko L et al (2014) Perinatal hypoxia: different effects of the inhibitors of GABA transporters GAT-1 and GAT-3 on the initial velocity of [3H]GABA uptake by cortical, hippocampal and thalamic nerve terminals. Croat Med J 55:250–258
Sah P, Hestrin S, Nicoll RA (1989) Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science 246:815–818
Soldatkin O, Nazarova A, Krisanova N et al (2015) Monitoring of the velocity of high-affinity glutamate uptake by isolated brain nerve terminals using amperometric glutamate biosensor. Talanta 135:67–74
Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547
Suna W, Shchepakin D, Kalachev LV, Kavanaugh MP (2014) Glutamate transporter control of ambient glutamate levels. Neurochem Int 73:146–151
van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH (2008) Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav 90:135–147
Acknowledgments
We would like to thank Dr. A. Soldatkin for glutamate biosensor measurements. This work was supported by Science and Technology Center in Ukraine/National Academy of Sciences of Ukraine Project #6055; Projects of National Academy of Sciences of Ukraine within Programs: “Scientific Space Research”; “Sensors for medicine, ecology, industry, and technology“; “Fundamental problems of synthesis of new chemical substances” (Grant N12-14); “Molecular and Cellular Biotechnology for Medicine, Industry and Agriculture”; and Cedars Sinai Medical Center’s International Research and Innovation Management Program, the Association for Regional Cooperation in the Fields of Health, Science and Technology (RECOOP HST Association) and the participating Cedars–Sinai Medical Center–RECOOP Research Centers (CRRC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests exist.
Rights and permissions
About this article
Cite this article
Borisova, T., Borysov, A., Pastukhov, A. et al. Dynamic Gradient of Glutamate Across the Membrane: Glutamate/Aspartate-Induced Changes in the Ambient Level of l-[14C]glutamate and d-[3H]aspartate in Rat Brain Nerve Terminals. Cell Mol Neurobiol 36, 1229–1240 (2016). https://doi.org/10.1007/s10571-015-0321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0321-4