Abstract
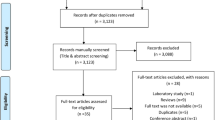
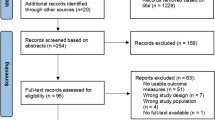
Symptoms of urogenital atrophy are common in breast cancer survivors. Its optimal management is currently unknown. A systematic review of randomized controlled trials (RCTs) evaluating treatments for urogenital atrophy in breast cancer patients was performed. EMBASE, Ovid Medline and the Cochrane Library were searched from 1946 to November 2014. Outcomes included improvements in both vaginal symptoms (e.g., dryness, pain, dyspareunia and itching) and vaginal hormone response measured by validated scales [e.g., Vaginal Health Index (VHI) and Vaginal Maturation Index (VMI)]. Of 430 unique citations identified, 4 studies (n = 196) met inclusion criteria. Interventions included pH-balanced gel, Replens®, lidocaine, Estring® and Vagifem®. Sample sizes ranged from 7 to 98 patients. Given the heterogeneity of the studies, a narrative synthesis of results was performed. One study of 98 patients suggested that vaginal pH-balanced gel (mean VHI 5.00 ± 0.816, mean VMI 51.18 ± 3.753) was more efficient than placebo (VHI 16.98 ± 3.875, p < 0.001, VMI 47.87 ± 2.728, p < 0.001) at 12 weeks in providing vaginal symptom relief. In patients who used lidocaine, 90 % had reduced dyspareunia compared to saline in a study of 46 patients. Although increased serum estradiol occurred, both Estring® and Vagifem® were shown to improve quality of life and VMI in a study of seven patients. Treatment of urogenital atrophy remains a challenging issue and there is a paucity of RCT evidence addressing this knowledge gap. It is evident that more prospective trials are needed.



Similar content being viewed by others
References
Sturdee DW, Panay N, International Menopause Society Writing Group (2010) Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 13(6):509–522
Sinha A, Ewies AA (2013) Non-hormonal topical treatment of vulvovaginal atrophy: an up-to-date overview. Climacteric 16(3):305–312
Ganz PA, Rowland JH, Meyerowitz BE et al (1998) Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res 152:396–411
Baumgart J, Nilsson K, Stavreus-Evers A et al (2011) Urogenital disorders in women with adjuvant endocrine therapy after early breast cancer. Am J Obstet Gynecol 204(1):26e1–26e7
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816
Suckling J, Lethaby A, Kennedy R (2006) Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 4:CD001500
Holmberg L, Anderson H (2004) HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet 363(9407):453–455
von Schoultz E, Rutgvist LE, Stockholm Breast Cancer Study Group (2005) Menopausal hormone therapy after breast cancer: the Stockholm randomized trial. J Natl Cancer Inst 97(7):533–535
Fahlen M, Fornander T, Johansson H et al (2013) Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer 49(1):52–59
Trinkaus M, Chin S, Wolfman W et al (2008) Should urogenital atrophy in breast cancer survivors be treated with topical estrogens? Oncologist 13(3):222–231
Chin SN, Trinkaus M, Simmons C et al (2009) Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 9(2):108–117
Gainford MC, Simmons C, Nguyen H et al (2005) A practical guide to the management of menopausal symptoms in breast cancer patients. Support Care Cancer 13(8):573–578
Bachmann G (1995) Urogenital ageing: an old problem newly recognized. Maturitas 22:S1–S5
Hess R, Austin RM, Dillon S et al (2008) Vaginal maturation index self-sample collection in mid-life women: acceptability and correlation with physician-collected samples. Menopause 15:726–729
Fallowfield LJ, Leaity SK, Howell A et al (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55(2):189–199
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:5928
Simmons CE, Kuchuk I, Freedman OC et al (2012) Are Estring(R) and Vagifem(R) equally effective and safe for the treatment of urogenital atrophy in breast cancer patients on aromatase inhibitor therapy? Clin Oncol (R Coll Radiol) 24(8):128–129
Goetsch MF, Lim JY, Caughey AB (2014) A solution for dyspareunia in breast cancer survivors. Obstet Gynecol 123(5):1S
Loprinzi CL, Abu-Ghazaleh S, Sloan JA et al (1997) Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol 15(3):969–973
Lee YK, Chung HH, Kim JW et al (2011) Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol 117(4):922–927
Modelska K, Cummings S (2002) Tibolone for postmenopausal women: systematic review of randomized trials. J Clin Endocrinol Metab 87(1):16–23
Kenemans P, Bundred NJ, Foidart JM et al (2009) Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 10(2):135–146
Sismondi P, Kimmings R, Kubista E et al (2011) Effects of tibolone on climacteric symptoms and quality of life in breast cancer patients—data from LIBERATE trial. Maturitas 70(4):365–372
Conflict of interest
We declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazzarello, S., Hutton, B., Ibrahim, M.F.K. et al. Management of urogenital atrophy in breast cancer patients: a systematic review of available evidence from randomized trials. Breast Cancer Res Treat 152, 1–8 (2015). https://doi.org/10.1007/s10549-015-3434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3434-z