Abstract
The herbal medicine, maoto, has been traditionally prescribed to patients with influenza in Japan. To better understand the efficacy of maoto for the treatment of influenza, a randomized trial was conducted for comparison with oseltamivir or zanamivir. Adult patients with influenza symptoms, including fever, positive for quick diagnostic kit for influenza within 48 h of fever onset were assessed for enrollment. The data of 28 patients randomly assigned to maoto (n = 10), oseltamivir (n = 8), or zanamivir (n = 10) were analyzed for the duration of fever (>37.5°C) and total symptom score from symptom cards recorded by the patient. Viral isolation and serum cytokine measurements were also done on days 1, 3, and 5. Maoto granules, a commercial medical dosage form, are made from four plants: Ephedra Herb, Apricot Kernel, Cinnamon Bark, and Glycyrrhiza Root. Median durations of fever of patients assigned maoto, oseltamivir, or zanamivir were 29, 46, or 27 h, respectively, significantly different for maoto and oseltamivir. No significant between-group differences were found in total symptom score among three groups. Viral persistent rates and serum cytokine levels (IFN-α, IL-6, IL-8, IL-10, and TNF-α) during the study period showed no differences among three groups. The administration of oral maoto granules to healthy adults with seasonal influenza was well tolerated and associated with equivalent clinical and virological efficacy to neuraminidase inhibitors.
Similar content being viewed by others
Introduction
The development of the currently available anti-influenza drugs, M2 proton channel inhibitors (amantadine and rimantadine) and neuraminidase inhibitors (oseltamivir and zanamivir), has made a remarkable contribution to the treatment of seasonal influenza [1]. These antivirals prevent the replication and spread of influenza virus, resulting in early recovery from flu symptoms. Neuraminidase inhibitors have also been effective for patients infected with pandemic influenza 2009. However, adverse effects, compliance problems, limited supply, and high cost are major concerns of neuraminidase inhibitors [1, 2]. In addition, the efficacy of these antivirals may be limited in the future as stable and transmissible drug-resistant strains emerge, as happened recently with A/H1N1 [3]. Thus, more attention is being paid to active, natural substances for influenza treatment, although current evidence tested by randomized trials is sparse and limited [4].
Traditional herbal medicines have played an important role in countries in the Far East, especially Japan, China, and Korea [5]. Kampo is a term for traditional herbal medicines that are commonly used in clinical practice throughout Japan. The principles of Kampo were originally based on traditional Chinese medicine, introduced to Japan about 1,500 years ago. Thereafter, Kampo established its own unique medical system, built up over the centuries through the clinical experiences of its practitioners [6]. In 1976, Kampo medical formulations were accepted by the national medical insurance system of Japan, allowing the widespread use of Kampo medicines. Maoto, one of these Kampo medicines, is prescribed mainly for acute febrile infectious diseases, such as colds, bronchitis, and influenza, although little is known about the precise pharmacological mechanisms. Recently, it was reported that maoto had a clinical antipyretic effect for children with seasonal influenza A [7] and that the duration of fever after treatment with maoto or maoto plus oseltamivir was significantly shorter than treatment with oseltamivir alone. In 2009 we also reported the efficacy of maoto for the treatment of adult seasonal influenza [8]. This report confirmed that maoto alone relieves the febrile response to influenza and the influenza symptoms as well as oseltamivir and that the antipyretic effect of maoto was remarkable at a very early stage of illness.
Symptoms and fever associated with influenza are related to viral replication and to a cytokine cascade response [9, 10]. Pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, interferon (IFN)-α, and tumor necrosis factor (TNF)-α, play important roles in both viral clearance and local inflammation. Several researchers have reported that local and systemic TNF-α and IL-6 levels are positively correlated to inflammatory reaction, such as viral load, influenza symptoms, and fever [11–14]. IFN-α is a key cytokine that inhibits the replication of influenza virus by inducing intracellular antiviral molecules, such as Mx GTPase, OAS, and PKR [15]. In the present study, we designed a randomized controlled trial, in a small group, to assess the efficacy of maoto on the flu symptoms and fever of patients with seasonal influenza and compared it with the modern antiviral drugs oseltamivir or zanamivir. We also assessed the viral persistence, serum cytokine concentration, and safety of these drugs.
Patients and methods
Patients
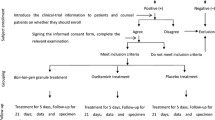
Eligible patients were aged 20–64 years, presented within 48 h of onset of flu symptoms, including fever (body temperature >38°C), and were positive by quick diagnostic test kit (Espline Influenza A & B-N; Fuji Rebio, Tokyo, Japan) for influenza virus antigens from nasal swabs. Patients with ischemic heart disease, hyperthyroidism, or prostate hypertrophy, or chronic infectious diseases, or who were receiving steroids, immunosuppressants, antivirals, or other herbal medicines, were excluded from this study. Sample size was designed to be 30 patients, considering the average number of influenza patients in past winter seasons in our clinic. During the study period (January–May 2009), subtypes A/H1N1, A/H3N2, and type B were epidemic. No patients with pandemic influenza A/H1N1 2009 were included in the groups studied. All patients provided written informed consent.
Study design
We followed the principles of the Declaration of Helsinki; the study protocol was designed as an open-labeled, randomized controlled trial according to the Consolidated Standards of Reporting Trial statement and was done in the influenza season (from January to May 2009) in the outpatient clinic of Fukuoka University Hospital. The institutional review board of Fukuoka University Hospital approved the study protocol (#12-10-08-75), and this protocol was uploaded on the Clinical Trial Registry (UMIN000001653) before the onset of patient enrolment in January 2009. The medical history, vital signs, physical examination data, age, sex, onset date/time of influenza symptoms, maximum body temperature, vaccination history, and duration from onset to study entry were recorded. Baseline virological and blood samples were collected before treatment. Patients were randomly assigned maoto, oseltamivir, or zanamivir. Randomization was performed by Clinical Support (Fukuoka, Japan) using a computer-generated system. The allocation method was concealed after the trial was completed.
The primary efficacy endpoint was the length of time from the start of medication to resolution of influenza illness (fever and symptoms). Secondary endpoints were viral persistence, safety, and serum cytokine concentration. For 5 days, each patient noted on a symptom card seven influenza symptom scores, body temperature three times a day (morning, afternoon, and at night), and the time of each dose of maoto, oseltamivir, or zanamivir. Symptoms were scored on a 4-point scale according to the severity of the symptom (0, none, to 3, severe). Symptoms were stuffy nose, rhinorrhea, sore throat, cough, myalgia/arthralgia, headache, and malaise. Total symptom score was the addition of each symptom score [9]. After 1 week, the symptom cards were mailed back to our hospital. The first time that a patient reported a fever of 37.5°C or higher was defined as the time of fever onset, according to the past reports [9, 16, 17]. The day on study entry was defined as day 1. The duration of fever and symptoms was recorded, the former from the time of the initial administration to becoming afebrile (<37.5°C) time and the latter from initial administration time to the time that total score was 7 or less. If the patients had a total score >7 on the last day, symptom duration was defined as hours from the time of the initial administration to the last recorded time. Adverse reactions were also recorded on the symptom cards.
Laboratory tests
Of the 28 participants, 18 were tested for blood counts and serum chemistry on days 1, 3, and 5 for the detection of adverse effects and cytokine measurement. Nasal swabs for virus isolation were also collected on days 1, 3, and 5. Serum specimens were stored at −80°C until the cytokine assay. Virus isolation was performed by standard methods using Madin–Darby canine kidney cells on specimen obtained from the nasal swabs. Subtype determination of influenza virus was performed by hemagglutinin inhibition (HAI) test with serum HAI antibodies (Denka Seiken, Tokyo, Japan).
For determination of serum cytokines, IL-1, IL-6, IL-8, IL-10, and TNF-α were measured simultaneously by bead-based flow cytometry (FACSCalibur; Becton-Dickinson Biosciences) using human cytokine bead array reagents (Bio-Plex; Bio-Rad, Hercules, CA, USA). In cytometric bead array measurement, different bead populations with distinct fluorescence intensities are coated with capturing antibodies specific for different cytokines. After incubation with 50 m serum, the beads are mixed with phycoerythrin-conjugated detection antibodies to form sandwich complexes. Fluorescence flow cytometry of the beads provides simultaneous quantification of a panel of cytokines. IL-1 concentration of all the samples was below the detection limit. Because of the low sensitivity in detection of serum IFN-α by cytokine bead array reagents, IFN-α level was determined by the bioactivity using luciferase-generated bioluminescence (iLite; Pestka Biomedical Laboratories, Piscataway, NJ, USA).
Drugs
Maoto is a multicomponent formulation extracted from four plants: Ephedra Herb, Apricot Kernel, Cinnamon Bark, and Glycyrrhiza Root. Maoto granules (Tsumura, Tokyo, Japan), a commercial medical dosage form, are made from the extracts through decoction, concentration, drying, and the addition of an excipient. Maoto was provided by Tsumura & Co. After being dissolved in warm water, Maoto granules were orally administered at 2.5 g TID for 5 days. Oseltamivir (Tamiflu; Roche) was orally administered at 75 mg BID for 5 days. Zanamivir (Relenza; GlaxoSmithKline) was dosed by inhalation at 20 mg BID for 5 days. No other drugs were coadministered, except acetaminophen (400 mg) in the case of high fever or severe headache. All patients started administration before 2 p.m. and took a full dose on day 1.
Statistical analysis
Between-group differences in symptom scores, body temperatures, and serum cytokine levels of the maoto, oseltamivir, and zanamivir groups were analyzed by Wilcoxon–Mann–Whitney test. Between-group differences of viral persistence rates in three groups were analyzed by chi-square test. P < 0.05 was considered significant.
Results
Patient characteristics
Forty-one eligible patients were assessed, and 33 were enrolled in the study (Fig. 1). Eight eligible patients refused to enter this study because the study was randomized controlled and could not allow the participants to choose anti-influenza drugs. They were also treated in our clinic using the standard neuraminidase inhibitors, and no severe case was found. Of 33 patients enrolled, 1 stopped taking the prescribed medication (oseltamivir) and 4 did not return their symptom cards; thus, the data of 28 patients (14 males; mean age, 28.7 years) were available for analysis. Samples for virus isolation and cytokine measurements were obtained from 18 patients. Patient characteristics are shown in Table 1. No significant differences were found in age, female to male ratio, or vaccination status. We followed the enrolled patients whether they showed any complications of influenza, such as sinusitis, pharyngitis, or pneumonia, but they did not show any complications throughout the study period. Median hours from fever onset to study entry were 17, 22, and 26 h in the maoto, oseltamivir, and zanamivir groups, respectively, with no significant between-group difference. Maximum body temperature before study entry was not significantly different among the three groups. Reduced peripheral lymphocyte number was reported to be a risk factor of serious complications of influenza [18]. Mean lymphocyte numbers (/μl) were 883, 842, and 997 in the maoto, oseltamivir, and zanamivir groups, respectively, with no significant difference.
All virus types in the maoto group were A by quick diagnostic test kit (Table 1). Virus isolation was performed for seven patients in the maoto group, four of whom were subtype H1N1 and three H3N2. In the oseltamivir group, two patients had type B and six type A (three were subtype H1N1 and one was H3N2 by virus isolation). In the zanamivir group, two patients had type B and eight type A (three were subtype H1N1 by virus isolation). Type B in the oseltamivir and zanamivir group was confirmed by virus isolation.
Duration of fever and symptoms
Duration of fever and symptoms from the initial administration is a useful parameter for the efficacy of anti-influenza drugs [19, 20]. The median duration of fever in patients assigned maoto, oseltamivir, or zanamivir was 29, 46, or 27 h, respectively (Fig. 2a). In the maoto group, duration of fever was significantly shorter than in the oseltamivir group. Median durations of symptoms represented by the total symptom scores of patients assigned maoto, oseltamivir, or zanamivir were 83, 87, or 94 h, respectively, with no significant differences (Fig. 2b). Maoto was identical to oseltamivir and zanamivir in its effectiveness in reducing the duration of fever and symptoms of patients with influenza.
Duration of fever and symptoms in influenza patients after the treatment with maoto, oseltamivir, or zanamivir. a Duration of fever, i.e., time of initial administration to afebrile (<37.5°C) time, plotted for each patient. b Duration of total symptom score, i.e., initial administration time to the time that total score was 7 or less. Horizontal bar indicates median hours
Time course of fever and total symptom score
For precise analysis, body temperature and total symptom score were plotted by time (Figs. 3, 4). The median body temperature of the three groups transiently increased at night on day 1, followed by a gradual decrease thereafter (Fig. 3a). There were no significant differences in median body temperature among the three groups at any point during the time course. The individual body temperature of patients treated with maoto is shown in Fig. 3b. Comparison with the oseltamivir (Fig. 3c) and zanamivir groups (Fig. 3d) showed that individuals in the maoto group were less febrile (37.5°C or more) in the latter phase of the time course. There were four individuals with fever on days 3, 4, and 5 (numbers 2, 3, 5, and 7 in Table 1) in the oseltamivir group, and three (numbers 3, 4, and 8) in the zanamivir group, but there was only one febrile patient (number 9) in the maoto group on days 3, 4, and 5. The median total symptom score of the three groups gradually reduced by day 5 (Fig. 4a), with no significant differences at any point during the time course (Fig. 4b–d).
Time course of body temperatures in influenza patients during treatment with maoto, oseltamivir, or zanamivir. Each patient recorded body temperatures three times per day (in morning, afternoon, and at night) for 5 days on a symptom card. Maoto, oseltamivir, or zanamivir was administered from day 1 to day 5. Median body temperature in patients assigned maoto, oseltamivir, or zanamivir (a). Individual body temperature in patients treated with maoto (b), oseltamivir (c), or zanamivir (d)
Time course of total symptom score in influenza patients during treatment with maoto, oseltamivir, or zanamivir. Symptoms (stuffy nose, rhinorrhea, sore throat, cough, myalgia/arthralgia, headache, malaise) were scored on a 4-point scale according to severity of the symptom (0, none, to 3, severe). Each patient recorded the symptom score three times per day (in morning, afternoon, and at night) for 5 days. Total symptom score was the addition of each symptom score. Maoto, oseltamivir, or zanamivir was administered from day 1 to day 5. Median total symptom score in patients assigned maoto, oseltamivir, or zanamivir (a). Individual total symptom scores in patients treated with maoto (b), oseltamivir (c), or zanamivir (d)
Viral persistence
To investigate the antiviral effect of the three drugs, we took nasal specimens containing influenza virus for virus isolation assay before and after the treatment (Table 2). Virus sampling was done for some of the patients of each group on days 1, 3, and 5. On day 3, virus was positive for four of seven patients (57.1%) in the maoto group, two of six (33.3%) in the oseltamivir group, and three of five (60%) in the zanamivir group. On day 5, virus was positive for one of seven patients (14.3%) in the maoto group, one of six (16.7%) in the oseltamivir group, and one of five (20.0%) in the zanamivir group. No significant between-group difference was found in the viral persistence rate on days 1, 3, or 5.
Serum cytokine concentration
Pro-inflammatory cytokines in response to influenza virus infection were closely related to influenza symptoms and viral clearance. We next examined the serum concentration of IFN-α, IL-6, IL-8, IL-10, and TNF-α before and after treatment to determine if cytokines involved in influenza virus infection were differently influenced by maoto than by neuraminidase inhibitors (Fig. 5). IFN-α, one of the most critical cytokines in viral clearance, was increased in all groups on day 1, and gradually reduced over time. No significant differences were found among the three groups. IL-6 and IL-10 levels were also increased in all groups on day 1, and gradually reduced over time. The TNF-α and IL-8 levels of the groups did not change significantly throughout the study period. The serum cytokine study in this study did not show remarkable differences among the three groups.
Serum cytokine response in influenza patients treated with maoto, oseltamivir, or zanamivir. Sera from patients assigned maoto (n = 7), oseltamivir (n = 6), or zanamivir (n = 5) on days 1, 3, and 5 were measured for interferon (IFN)-α, interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α. Maoto, oseltamivir, or zanamivir was administered from day 1 to day 5. Each dot indicates median level of serum cytokine
Safety
One patient in the maoto group (number 9) and one in the oseltamivir group (number 4) showed a mildly elevated serum aminotransferase level after treatment (data not shown). The level of aminotransferase of these patients was reduced to normal within 2 weeks. No other adverse reactions were seen.
Discussion
The administration of oral maoto granules was associated with significant clinical efficacy for healthy adults with naturally occurring influenza and was generally well tolerated. The efficacy is equivalent to oseltamivir and zanamivir, the most commonly used neuraminidase inhibitors in Japan. Our previous study, not randomized, also showed the effectiveness of maoto for influenza, but only showed differences between the background of study groups prescribed maoto and oseltamivir [8]. In addition, the virological analysis of the present study was designed to investigate the viral persistence rate of patients with influenza. Through these two studies, we confirmed that maoto granules have anti-influenza virus efficacy equal to these neuraminidase inhibitors, the standard anti-influenza drugs.
One of the major symptoms in influenza is fever. Maoto significantly shortened the duration of fever in comparison with oseltamivir and was approximately equal to zanamivir in effect. This influenza was not caused by the recently emerged subtype of A/H1N1 that is resistant to oseltamivir treatment [21–23], none of which was found in the study period in Japan. However, two patients with a duration of fever >80 h in the oseltamivir group were infected with influenza B (patients 3 and 5). It has been reported that the antiviral effect of oseltamivir to influenza B is relatively low compared with that of zanamivir [17, 24]. It is a major concern whether maoto would have antiviral efficacy against influenza B. Alleviation of influenza symptoms (stuffy nose, rhinorrhea, sore throat, cough, myalgia/arthralgia, headache, and malaise) was not different among the three groups. Because patients with typical influenza not taking medication suffer from high fever and flu-like symptoms for 3–7 days, these drugs were obviously effective. It is possible that maoto is as effective as nonsteroidal antiinflammatory drugs against influenza symptoms. If maoto had few or no antiviral effects, patients assigned maoto would have shed influenza virus for longer periods, but our viral persistence examination of clinical isolates on days 3 and 5 showed that maoto had an antiviral effect similar to that of neuraminidase inhibitors.
The Japanese traditional herbal medicines included in Kampo are characterized by drugs made of the extracts of several boiled plants. Kampo was originally introduced from ancient China and has been developed over many centuries in Japan [5, 6, 25]. Maoto is traditionally prescribed for acute febrile diseases, such as influenza, measles, typhoid fever, and pneumonia. It was reported to have been used in Japan during the ‘Spanish Flu’ pandemic and to have had some efficacy [26]. It is interesting that maoto is effective for several acute infectious diseases caused by a variety of viruses, although neuraminidase inhibitors and M2 proton channel inhibitors are specific for influenza virus infection. Unfortunately, because there are few modern clinical trials in herbal medicines for influenza [4, 27], maoto has not been able to generate the general consensus necessary to build a worldwide following as an anti-influenza drug. Maoto consists of four plants: Ephedra Herb, Apricot Kernel, Cinnamon Bark, and Glycyrrhiza Root. Recently, a Chinese research group reported the efficacy of antiwei, a traditional Chinese herbal medicine, in a randomized, double-blind, placebo-controlled trial [28]. Antiwei is surprisingly similar to maoto. It is made of seven plants, including the four plants comprising maoto. Maoto has also been reported to have an antipyretic effect in the treatment of children with influenza A [7]. These studies provide a rationale for continuing the study of traditional herbal medicines as anti-influenza drugs. Economically, these herbal drugs are generally of low cost; In Japan, maoto costs about ten times less than neuraminidase inhibitors over 5 days of treatment.
The major component of Ephedra Herb is ephedrine, which activates both the sympathetic and central nervous systems and hence may produce unwanted symptoms such as insomnia, palpitation, rapid pulse, elevation of blood pressure, and dysuria [29]. Maoto is not recommended for patients with ischemic heart diseases, hyperthyroidism, or prostate hypertrophy or for the frail elderly. Short-term administration (3–5 days) is necessary to avoid the harmful effects of Ephedra. The protocol of the present study excluded patients with these conditions.
It is not known precisely how maoto acts on influenza. The life cycle of the influenza virus is classified into several steps: binding to the host cell membrane, fusion and uncoating, replication, translation, assembly, and release of virion. It is known that anti-influenza virus drugs inhibit this cycle at some point [30]. In vitro, Mantani et al. [31] reported that the Ephedra Herb inhibits the fusion of influenza A virus to the cell membrane through the inhibition of acidification of endosomes. Maoto may act on several points of the virus life cycle because it is a multicomponent formulation that includes three herbal plants in addition to Ephedra Herb. It is also possible that maoto may act on the induction of pro-inflammatory cytokines that play critical roles in virus eradication in the early phase of influenza, through the induction of intracellular antiviral molecules. Several pro-inflammatory cytokines, such as IL-1, IL-6, IL-8, IFN-α, and TNF-α, are produced after the pattern recognition receptors (PRR) in host cells after stimulation by influenza virus, whereas IL-10 inhibits these pro-inflammatory cytokines [9–11, 32, 33]. IFN-α is a key cytokine in the inhibition of virus replication through the induction of intracellular antiviral molecules, such as Mx GTPase, OAS, and PKR [15]. In severe cases of influenza, such as avian influenza, attenuated IFN-α response may contribute to the pathogenesis [34, 35]. TNF-α and IL-6 have been detected in patient sera and nasal lavage fluid and have correlated well with viral titer and symptom score [32]. In the present study, maoto did not increase or decrease the serum IFN-α, TNF-α, IL-6, IL-8, or IL-10 levels compared with oseltamivir and zanamivir, suggesting that maoto did not eradicate influenza virus through the induction of pro-inflammatory cytokines. In our in vitro experiment on influenza infection, maoto and oseltamivir did not elevate the level of pro-inflammatory cytokines (data not shown).
We recognize that this study has some potential limitations, such as small subject number and the exclusion of high-risk patients. In addition, maoto was not assigned to patients with influenza B virus infection, and the effectiveness of maoto to influenza 2009 pdm was not confirmed. Further large-scale studies are needed to investigate the effectiveness of maoto against other influenza subtypes, for patients at high risk, children, and influenza-related pneumonia. To better understand the antiviral mechanism(s) of maoto, we are preparing in vitro experiments using maoto with human cell lines infected with influenza virus.
References
Hayden FG. Antivirals for influenza: historical perspectives and lessons learned. Antiviral Res. 2006;71:372–8.
Ong AK, Hayden FG, John F. Enders lecture 2006: antivirals for influenza. J Infect Dis. 2007;196:181–90.
Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48(suppl 1):S3–13.
Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza-like illness. Am J Med. 2007;120(923–9):e3.
Nishimura K, Plotnikoff GA, Watanabe K. Kampo medicine as an integrative medicine in Japan. JMAJ. 2009;52:147–9.
Ishibashi A, Kosoto H, Ohno S, Sakaguchi H, Yamada T, Matsuda K. General introduction to Kampo. In: Sato Y, editor. Introduction to Kampo. Tokyo: Elsevier Japan; 2005. p. 2–15.
Kubo T, Nishimura H. Antipyretic effect of Mao-to, a Japanese herbal medicine, for treatment of type A influenza infection in children. Phytomedicine. 2007;14:96–101.
Nabeshima S, Kashiwagi K, Ajisaka K, Kitajima K, Masui S, Ikematsu H, et al. A comparison of oseltamivir with maoto, a traditional herbal medicine, for the treatment of adult seasonal influenza A. J Tradit Med. 2010;27:148–56.
Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–6.
Fritz RS, Hayden FG, Calfee DP, Cass LM, Peng AW, Alvord WG, et al. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J Infect Dis. 1999;180:586–93.
Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–8.
Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat Med. 2002;8:950–4.
Van Reeth K, Van Gucht S, Pensaert M. Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza virus challenge of vaccination-immune pigs. Viral Immunol. 2002;15:583–94.
Lee N, Wong CK, Chan PK, Lun SW, Lui G, Wong B, et al. Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza A virus infection. Clin Infect Dis. 2007;45:723–31.
Boo KH, Yang JS. Intrinsic cellular defenses against virus infection by antiviral type I interferon. Yonsei Med J. 2010;51:9–17.
Kawai N, Ikematsu H, Iwaki N, Kawamura K, Kawashima T, Kashiwagi S. A change in the effectiveness of amantadine for the treatment of influenza over the 2003–2004, 2004–2005, and 2005–2006 influenza seasons in Japan. J Infect Chemother. 2007;13:314–9.
Kawai N, Ikematsu H, Iwaki N, Maeda T, Kanazawa H, Kawashima T, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect. 2008;56:51–7.
Nabeshima S, Murata M, Kikuchi K, Ikematsu H, Kashiwagi S, Hayashi J. A reduction in the number of peripheral CD28+CD8+T cells in the acute phase of influenza. Clin Exp Immunol. 2002;128:339–46.
Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Maeda T, et al. Factors influencing the effectiveness of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002–2003 influenza season. Clin Infect Dis. 2005;40:1309–16.
Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, et al. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006;43:439–44.
Kawai N, Ikematsu H, Iwaki N, Kondou K, Hirotsu N, Kawashima T, et al. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J Infect. 2009;59:207–12.
Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034–41.
Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953–6.
Kawai N, Ikematsu H, Iwaki N, Maeda T, Kawashima T, Hirotsu N, et al. Comparison of the effectiveness of Zanamivir and Oseltamivir against influenza A/H1N1, A/H3N2, and B. Clin Infect Dis. 2009;48:996–7.
Terasawa K. Evidence-based Reconstruction of Kampo Medicine: Part I-Is Kampo CAM? Evid Based Complement Altern Med. 2004;1:11–6.
Palmer E, Rice GW. Divine wind versus devil wind: popular response to pandemic influenza in Japan, 1918–1919. Jpn Forum. 1992;4:317–28.
Wang X, Jia W, Zhao A. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20:335–41.
Wang L, Zhang RM, Liu GY, Wei BL, Wang Y, Cai HY, et al. Chinese herbs in treatment of influenza: a randomized, double-blind, placebo-controlled trial. Respir Med. 2010;104:1362–9.
Xu FH, Uebaba K. Effect of Kampo formulations (traditional Chinese medicine) on circulatory parameters. Acupunct Electrother Res. 1999;24:11–28.
Das K, Aramini JM, Ma LC, Krug RM, Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol. 2010;17:530–8.
Mantani N, Andoh T, Kawamata H, Terasawa K, Ochiai H. Inhibitory effect of Ephedrae herba, an oriental traditional medicine, on the growth of influenza A/PR/8 virus in MDCK cells. Antiviral Res. 1999;44:193–200.
Gentile D, Doyle W, Whiteside T, Fireman P, Hayden FG, Skoner D. Increased interleukin-6 levels in nasal lavage samples following experimental influenza A virus infection. Clin Diagn Lab Immunol. 1998;5:604–8.
Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–9.
Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, et al. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type i interferon response in polarized human bronchial epithelial cells. J Virol. 2007;81:12439–49.
Hayman A, Comely S, Lackenby A, Hartgroves LC, Goodbourn S, McCauley JW, et al. NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response. J Virol. 2007;81:2318–27.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nabeshima, S., Kashiwagi, K., Ajisaka, K. et al. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J Infect Chemother 18, 534–543 (2012). https://doi.org/10.1007/s10156-012-0378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0378-7