Abstract
Background
The 5-aminolevulinic acid (5-ALA) fluorescence-guided resection of recurrent malignant glioma is a standard surgical procedure at many neuro-oncological centers and is considered to be equally reliable as the primary resection of these tumors. 5-ALA induced fluorescence (5-AIF)-guided resection has been demonstrated to be highly predictive for tumor tissue. As pseudoprogression and radiation-induced necrosis are critical differential diagnoses of glioma recurrence, the purpose of the present analysis was to analyze 5-AIF behavior in resected tissue specimens histopathologically showing regressive and reactive changes but lacking active, that is, cellular recurrent tumor tissue after adjuvant treatment of malignant glioma.
Methods
A retrospective analysis was performed in patients suffering from malignant glioma who underwent surgical resection for suspected contrast-enhancing tumor recurrence (according to RANO criteria) at our institution between 2007 and 2013, but in whom histopathological analysis only revealed reactive changes. The presence of AIF in the resected tissue samples was intraoperatively assessed and classified by the surgeon, using the categories (1) no, (2) vague and (3) solid AIF.
Results
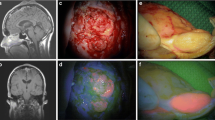
A total of 13 out of 313 patients who underwent AIF-guided surgical resection of tissue suspicious for recurrent glioma histologically demonstrated only reactive changes without active recurrent tumor tissue after adjuvant therapy. Pretreatment was chemotherapy with temozolomide in 1 patient and combined radio-/chemotherapy in 12 patients. Six patients had suffered previous tumor recurrence with a subsequently intensified adjuvant therapy. Seven of the 13 patients displayed solid, 5 patients vague and 1 patient no 5-AIF of the resected tissue specimens. However, all 5-AIF-positive lesions exhibited heterogeneous fluorescence patterns with vaguely or solidly fluorescent as well as nonfluorescent regions.
Conclusions
Resection of reactive tissue without active recurrent tumor after multimodal treatment for glioblastoma is frequently associated with solid or vague 5-AIF. Therefore, neurosurgeons should remain cautious when attempting to employ intraoperative 5-AIF to discriminate radiation- and chemotherapy-induced tissue changes from true disease progression. Nevertheless, 5-AIF-guided resection remains a valid tool in the neurosurgical treatment of recurrent gliomas.


Similar content being viewed by others
References
Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, Franceschi E (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10:361–367
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82:81–83
Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H (2009) Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: Clinical observations and working recommendations. Surg Neurol 72:423–428
de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ (2004) Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63:535–537
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9:180–188
Gunjur A, Lau E, Taouk Y, Ryan G (2011) Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: A retrospective analysis. J Med Imaging Radiat Oncol 55:603–610
Kamp MA, Dibue M, Niemann L, Reichelt DC, Felsberg J, Steiger HJ, Szelenyi A, Rapp M, Sabel M (2012) Proof of principle: Supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir (Wien) 154:1981–1986
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G, Sabel M (2012) 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir (Wien) 154:223–228, discussion 228
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) WHO classification of tumors of the central nervous system. Acta Neuropathol 114(2):97–109
Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M, Group ALA-RGS (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 65:1070–1076, discussion 1076-1077
Perry A, Schmidt RE (2006) Cancer therapy-associated CNS neuropathology: An update and review of the literature. Acta Neuropathol 111:197–212
Stummer W, Kamp MA (2009) The importance of surgical resection in malignant glioma. Curr Opin Neurol 22:645–649
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEJM 352:987-996
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van Es CA, van den Bent MJ (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y, Kondo K, Miyajima Y, Fujii K (2007) Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo) 47:210–213, discussion 213-214
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Wong CS, Van der Kogel AJ (2004) Mechanisms of radiation injury to the central nervous system: Implications for neuroprotection. Mol Interv 4:273–284
Woodworth GF, Garzon-Muvdi T, Ye X, Blakeley JO, Weingart JD, Burger PC (2013) Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol 113:485–493
Conflicts of interest
G. Reifenberger has received honoraria for lectures and from advisory boards from Amgen, Roche and Meck Serono as well as research funding from Roche. The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Among 313 patients operated on for recurrent malignant gliomas in whom recurrences were diagnosed from contrast-enhancing tumor (as diagnosed by the RANO criteria), the authors did not find active tumor in 13 patients (3 %) despite visible fluorescence. According to the authors, fluorescing tissues demonstrated regressive changes associated with radiotherapy or radio-chemotherapy, such as radionecrosis or reactive gliosis, but not normal brain. Thus, this report on a large cohort of patients operated on for recurrent gliomas using 5-ALA confirms earlier findings by Nabavi et al. (2009) who also found 3 % of samples did not show viable tumor tissue, but not normal brain in 40 patients operated on for recurrences. On the other hand, this observation implies 5-ALA to be accurate 97 % of the time in pretreated patients, which is unquestionably reassuring.
I completely agree with the authors that 5-ALA is therefore a very helpful tool for recurrent malignant glioma surgery. However, surgeons should be aware of a small number of patients diagnosed with recurrent gliomas that actually have radiation necrosis or other treatment-induced changes, rather than active tumor, which might also show some degree of fluorescence. Fortunately, even if such areas are resected, they will not harbor normal brain.
The authors' experience is a valuable contribution to the literature on fluorescence-guided resections using 5-ALA, and they are to be commended on their large series.
Walter Stummer
Münster, Germany
Rights and permissions
About this article
Cite this article
Kamp, M.A., Felsberg, J., Sadat, H. et al. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir 157, 207–214 (2015). https://doi.org/10.1007/s00701-014-2313-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2313-4