Abstract
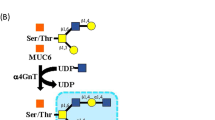
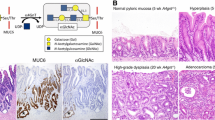
Gastric gland mucin-specific O-glycans are unique in having α1,4-linked N-acetylglucosamine (αGlcNAc) attached to MUC6. We previously reported decreased expression of αGlcNAc relative to MUC6 in gastric and pancreatic neoplasms, but its significance in cervical glandular lesions remained unclear. Here, we analyzed MUC5AC, MUC6, αGlcNAc, and p16 expression in 9 lesions of mucinous carcinoma, gastric type with minimal deviation adenocarcinoma (GAS-MDA), 5 of GAS with nonMDA (GAS-nonMDA), 14 of typical lobular endocervical gland hyperplasia (LEGH), and 5 of atypical LEGH (33 total lesions). All 33 were MUC5AC-positive. Moreover, all 14 typical LEGH, 5 atypical LEGH, 8 of 9 GAS-MDA, and 3 of 5 GAS-nonMDA were MUC6-positive. All 14 typical LEGH, 2 of 5 atypical LEGH, 3 of 9 GAS-MDA, and 1 of 5 GAS-nonMDA were αGlcNAc-positive. The proportion of αGlcNAc-positive atypical LEGH or GAS-MDA or GAS-nonMDA lesions was significantly smaller than that seen in typical LEGH lesions (P < 0.001 and P < 0.01, respectively). Of 33 lesions, 32 were p16-negative. Furthermore, when we evaluated MUC6 and αGlcNAc immunoreactivity semi-quantitatively in all 33 lesions, in typical LEGH and GAS-MDA, the immunohistochemical score for αGlcNAc was significantly lower than that for MUC6 (P < 0.01). We did not observe significantly decreased αGlcNAc expression relative to MUC6 in typical LEGH lesions. These studies suggest that αGlcNAc expression decreases as typical LEGH progresses to GAS. Given the difficulty in distinguishing MDA and atypical LEGH from typical LEGH in H.E. staining, we propose that immunohistochemical analysis of αGlcNAc and MUC6 could be useful.


Similar content being viewed by others
References
Wells M, Ostor AG, Crum CP et al (2003) Epithelial tumours. In: Tavassoli FA, Devilee P (eds) Pathology and genetics of tumours of the breast and female genital organs. International Agency for Reserch on Cancer, Lyon, pp 262–273
Smith HO, Tiffany MF, Qualls CR, Key CR (2000) The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol 78:97–105. https://doi.org/10.1006/gyno.2000.5826
Walboomers JM, Jacobs MV, Manos MM et al (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. https://doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998) Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 153:1741–1748. https://doi.org/10.1016/S0002-9440(10)65689-1
Zappa M, Visioli CB, Ciatto S, Lossa A, Paci E, Sasieni P (2004) Lower protection of cytological screening for adenocarcinomas and shorter protection for younger women: the results of a case-control study in Florence. Br J Cancer 90:1784–1786. https://doi.org/10.1038/sj.bjc.6601754
Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, Nishimura R (2007) Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 31:664–672. https://doi.org/10.1097/01.pas.0000213434.91868.b0
Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC (2011) Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol 35:633–646. https://doi.org/10.1097/PAS.0b013e31821534b9
Kurman RJ, Norris HJ, Wilkinson E (1992) Tumors of the cervix. In: Rosai J, Sobin LH (eds) Atlas of tumor pathology, tumors of the cervix, vagina and vulva, 3rd series edn. AFIP, Washington, DC, pp 91–93
Wright TC, Ferenczy AKJ (2011) Blaustein’s pathology of the female genital tract, 6th edn. Springer-Verlag, New York
Nucci MR, Clement PB, Young RH (1999) Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol 23:886–891
Mikami Y, Hata S, Melamed J, Fujiwara K, Manabe T (2001) Lobular endocervical glandular hyperplasia is a metaplastic process with a pyloric gland phenotype. Histopathology 39:364–372. https://doi.org/10.1046/j.1365-2559.2001.01239.x
Ishii K, Ota H, Katsuyama T (2000) Lobular endocervical glandular hyperplasia represents pyloric gland metaplasia? Am J Surg Pathol 24(325):323
Mikami Y, McCluggage WG (2013) Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol 20:227–237. https://doi.org/10.1097/PAP.0b013e31829c2d66
Ishii K, Hosaka N, Toki T, Momose M, Hidaka E, Tsuchiya S, Katsuyama T (1998) A new view of so-called adenoma malignum of the uterine cervix. Virchows Arch 432:315–322
Wilbur DC, Mikami Y, Colgan TJ et al (2014) Tumors in uterine cervix. Glandular tumours and precursors. In: WHO classification of tumours of the female reproductive organs, 4th edn. IARC Press, Lyon, pp 184–192
Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y (1991) A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J 23:22–28
Ishihara K, Kurihara M, Goso Y et al (1996) Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318:409–416
Shiratsu K, Higuchi K, Nakayama J (2014) Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Sci 105:126–133. https://doi.org/10.1111/cas.12305
Yamanoi K, Sekine S, Higuchi K, Kushima R, Nakayama J (2015) Decreased expression of gastric gland mucin-specific glycan α1,4-linked N-acetylglucosamine on its scaffold mucin 6 is associated with malignant potential of pyloric gland adenoma of the stomach. Histopathology 67:898–904. https://doi.org/10.1111/his.12728
Zhang MX, Nakayama J, Hidaka E, Kubota S, Yan J, Ota H, Fukuda M (2001) Immunohistochemical demonstration of α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ residues in human gastrointestinal mucosa. J Histochem Cytochem 49:587–596. https://doi.org/10.1177/002215540104900505
Yamanoi K, Nakayama J (2018) Reduced αGlcNAc glycosylation on gastric gland mucin is a biomarker of malignant potential for gastric cancer, Barrett’s adenocarcinoma, and pancreatic cancer. Histochem Cell Biol. https://doi.org/10.1007/s00418-018-1667-8
Nakayama J, Yeh JC, Misra AK, Ito S, Katsuyama T, Fukuda M (1999) Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci U S A 96:8991–8996 http://www.pnas.org/content/96/16/8991.long
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 122:923–934. https://doi.org/10.1172/JCI59087
Matsuzawa K, Akamatsu T, Katsuyama T (1992) Mucin histochemistry of pancreatic duct cell carcinoma, with special reference to organoid differentiation simulating gastric pyloric mucosa. Hum Pathol 23:925–933. https://doi.org/10.1016/0046-8177(92)90407-T
McCluggage WG, Harley I, Houghton JP et al (2010) Composite cervical adenocarcinoma composed of adenoma malignum and gastric type adenocarcinoma (dedifferentiated adenoma malignum) in a patient with Peutz Jeghers syndrome. J Clin Pathol 63:935–941. https://doi.org/10.1136/jcp.2010.080150
Mikami Y, Kiyosawa T, Hata S et al (2004) Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/puloric gland metaplasia and ‘adenoma malignum’. Mod Pathol 17:962–972. https://doi.org/10.1038/modpathol.3800148
Matsubara A, Sekine S, Ogawa R, Yoshida M, Kasamatsu T, Tsuda H, Kanai Y (2014) Lobular endocervical glandular hyperplasia is a neoplastic entity with frequent activating GNAS mutations. Am J Surg Path 38:370–376. https://doi.org/10.1097/PAS.0000000000000093
Ohya A, Yamanoi K, Shimojo H, Fujii C, Nakayama J (2017) Gastric gland mucin-specific O-glycan expression decreased with tumor progression from precursor lesions to pancreatic cancer. Cancer Sci 108:1897–1902. https://doi.org/10.1111/cas.13317
Nishiwaki M, Yamamoto T, Tone S, Murai T, Ohkawara T, Matsunami T, Koizumi M, Takagi Y, Yamaguchi J, Kondo N, Nishihira J, Horikawa T, Yoshiki T (2008) Genotyping of human papillomaviruses by a novel one-step typing method with multiplex PCR and clinical applications. J Clin Microbiol 46:1161–1168. https://doi.org/10.1128/JCM.00793-07
Ohta Y, Suzuki T, Hamatani S, Shiokawa A, Kushima M, Ota H (2008) Lobular endocervical glandular hyperplasia might become a precursor of adenocarcinoma with pyloric gland features. Pathol Res Pract 204:683–687. https://doi.org/10.1016/j.prp.2008.02.009
Chang HJ, Kim SW, Lee BL, Hong EK, Kim WH (2004) Phenotypic alterations of mucins and cytokeratins during gallbladder carcinogenesis. Pathol Int 54:576–584. https://doi.org/10.1111/j.1440-1827.2004.01666.x
Matsukita S, Nomoto M, Kitajima S et al (2003) Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of Breast: comparison with invasive ductal carcinoma. Histopathology 42:26–36. https://doi.org/10.1046/j.1365-2559.2003.01530.x
Acknowledgements
The authors thank Dr. Elise Lamar for editing the manuscript.
Founding
This work was supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (nos. 17K15640 (K.Y.) and 15H04712 (J.N.)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the ethics committee of Shinshu University School of Medicine, Japan (project no. 3737 was approved on July 4, 2017).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yamanoi, K., Ishii, K., Tsukamoto, M. et al. Gastric gland mucin-specific O-glycan expression decreases as tumor cells progress from lobular endocervical gland hyperplasia to cervical mucinous carcinoma, gastric type. Virchows Arch 473, 305–311 (2018). https://doi.org/10.1007/s00428-018-2381-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2381-6