Abstract
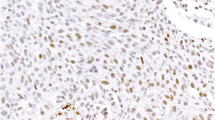
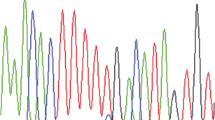
Pancreatic acinar cell carcinomas (PACs) are rare but are distinct aggressive neoplasms that phenotypically differ from pancreatic ductal adenocarcinomas (PDACs) and pancreatic neuroendocrine neoplasms (PNENs). Despite recent work on the genetic changes of PACs, their molecular pathogenesis is still poorly understood. In this study, we focus on a comparative genomic hybridization analysis. Based on frequent chromosomal imbalances, the involvement of DCC and c-MYC in the pathogenesis of PACs is further investigated. Moreover, we examine markers harboring potential therapeutic relevance (K-RAS, BRAF, EGFR, MGMT, HSP90, L1CAM, Her2). PACs revealed a microsatellite stable, chromosomal unstable genotype, defined by recurrent chromosomal losses of 1p, 3p, 4q, 5q, 6q, 8p, 9p, 11q, 13q, 16q, and 18, as well as gains of 1q, 7, 8q, 12, 17q, and 20q. Subsets of PAC displayed reduction/loss of DCC (79 %) and c-MYC-amplification (17 %). Significant EGFR expression occurred in 42 %, HSP90 expression in 98 %, L1CAM expression in 72 %, and loss of MGMT in 26 %. Two cases carried a K-RAS mutation. Mutations of EGFR or BRAF were not detected. All cases were Her2/neu-negative. PACs display characteristic chromosomal imbalances which are distinctly different from those in pancreatic ductal adenocarcinomas and pancreatic neuroendocrine neoplasms. Our findings suggest that DCC and c-MYC alterations may play an important role in the pathogenesis of PACs. Furthermore, EGFR, MGMT, HSP90, and L1CAM may be useful as therapeutic markers and predictors of response to therapy in a subset of PACs.





Similar content being viewed by others
References
Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS (2008) 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 144(2):141–148. doi:10.1016/j.surg.2008.03.006
Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY (2008) Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg 12(12):2078–2086. doi:10.1007/s11605-008-0705-6
Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M (2007) Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from pancreatic cancer registry of Japan pancreas society. Pancreas 35(1):42–46. doi:10.1097/mpa.0b013e31804bfbd3
Klimstra DS, Heffess CS, Oertel JE, Rosai J (1992) Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 16(9):815–837
Osborne BM, Culbert SJ, Cangir A, MacKay B (1977) Acinar cell carcinoma of the pancreas in a 9-year-old child: case report with electron microscopic observations. South Med J 70(3):370–372
Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, O’Reilly EM (2011) Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist 16(12):1714–1720. doi:10.1634/theoncologist.2011-0231
Klimstra DS, Hruban RH, Klöppel G, Morohoshi T, Ohike N (2010) Acinar cell neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system. International Agency for Research on Cancer, Lyon, pp 314–318
La Rosa S, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, Marando A, Notohara K, Sessa F, Vanoli A, Zhang L, Capella C (2012) Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol 36(12):1782–1795. doi:10.1097/PAS.0b013e318263209d
Colombo P, Arizzi C, Roncalli M (2004) Acinar cell cystadenocarcinoma of the pancreas: report of rare case and review of the literature. Hum Pathol 35(12):1568–1571
Stelow EB, Shaco-Levy R, Bao F, Garcia J, Klimstra DS (2010) Pancreatic acinar cell carcinomas with prominent ductal differentiation: Mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol 34(4):510–518. doi:10.1097/PAS.0b013e3181cfcac7
Ohike N, Kosmahl M, Kloppel G (2004) Mixed acinar-endocrine carcinoma of the pancreas A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch 445(3):231–235. doi:10.1007/s00428-004-1037-x
Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS (2002) Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol 160(3):953–962
Rigaud G, Moore PS, Zamboni G, Orlandini S, Taruscio D, Paradisi S, Lemoine NR, Kloppel G, Scarpa A (2000) Allelotype of pancreatic acinar cell carcinoma. Int J Cancer 88(5):772–777
Terhune PG, Heffess CS, Longnecker DS (1994) Only wild-type c-Ki-ras codons 12, 13, and 61 in human pancreatic acinar cell carcinomas. Mol Carcinog 10(2):110–114
Hoorens A, Lemoine NR, McLellan E, Morohoshi T, Kamisawa T, Heitz PU, Stamm B, Ruschoff J, Wiedenmann B, Kloppel G (1993) Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and Ki-ras mutation. Am J Pathol 143(3):685–698
Pellegata NS, Sessa F, Renault B, Bonato M, Leone BE, Solcia E, Ranzani GN (1994) K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res 54(6):1556–1560
Moore PS, Orlandini S, Zamboni G, Capelli P, Rigaud G, Falconi M, Bassi C, Lemoine NR, Scarpa A (2001) Pancreatic tumours: molecular pathways implicated in ductal cancer are involved in ampullary but not in exocrine nonductal or endocrine tumorigenesis. Br J Cancer 84(2):253–262. doi:10.1054/bjoc.2000.1567
de Wilde RF, Ottenhof NA, Jansen M, Morsink FH, de Leng WW, Offerhaus GJ, Brosens LA (2011) Analysis of LKB1 mutations and other molecular alterations in pancreatic acinar cell carcinoma. Mod Pathol 24(9):1229–1236. doi:10.1038/modpathol.2011.83
Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang CL, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N, Wood LD (2014) Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol 232(4):428–435. doi:10.1002/path.4310
Furlan D, Sahnane N, Bernasconi B, Frattini M, Tibiletti MG, Molinari F, Marando A, Zhang L, Vanoli A, Casnedi S, Adsay V, Notohara K, Albarello L, Asioli S, Sessa F, Capella C, La Rosa S (2014) APC alterations are frequently involved in the pathogenesis of acinar cell carcinoma of the pancreas, mainly through gene loss and promoter hypermethylation. Virchows Arch. doi:10.1007/s00428-014-1562-1
Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, Yeo CJ, Cameron JL, Hruban RH (2001) Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol 159(5):1619–1627
Kitoh H, Ryozawa S, Harada T, Kondoh S, Furuya T, Kawauchi S, Oga A, Okita K, Sasaki K (2005) Comparative genomic hybridization analysis for pancreatic cancer specimens obtained by endoscopic ultrasonography-guided fine-needle aspiration. J Gastroenterol 40(5):511–517. doi:10.1007/s00535-005-1577-0
Taruscio D, Paradisi S, Zamboni G, Rigaud G, Falconi M, Scarpa A (2000) Pancreatic acinar carcinoma shows a distinct pattern of chromosomal imbalances by comparative genomic hybridization. Genes Chromosomes Cancer 28(3):294–299
Rieker RJ, Aulmann S, Penzel R, Schnabel PA, Blaeker H, Esposito I, Morresi-Hauf A, Otto HF, Hecker E, Dienemann H, Schirmacher P, Mechtersheimer G (2005) Chromosomal imbalances in sporadic neuroendocrine tumours of the thymus. Cancer Lett 223(1):169–174. doi:10.1016/j.canlet.2004.10.027
Huszar M, Moldenhauer G, Gschwend V, Ben-Arie A, Altevogt P, Fogel M (2006) Expression profile analysis in multiple human tumors identifies L1 (CD171) as a molecular marker for differential diagnosis and targeted therapy. Hum Pathol 37(8):1000–1008. doi:10.1016/j.humpath.2006.03.014
Bergmann F, Aulmann S, Wente MN, Penzel R, Esposito I, Kleeff J, Friess H, Schirmacher P (2006) Molecular characterisation of pancreatic ductal adenocarcinoma in patients under 40. J Clin Pathol 59(6):580–584. doi:10.1136/jcp.2005.027292
Warth A, Penzel R, Brandt R, Sers C, Fischer JR, Thomas M, Herth FJ, Dietel M, Schirmacher P, Blaker H (2012) Optimized algorithm for Sanger sequencing-based EGFR mutation analyses in NSCLC biopsies. Virchows Arch 460(4):407–414. doi:10.1007/s00428-012-1219-x
Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, Dondog B, Pawlita M, Dippold W, Wagner R, Gebert J, von Knebel DM (2005) T25 repeat in the 3′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res 65(18):8072–8078. doi:10.1158/0008-5472.CAN-04-4146
Gunawan B, von Heydebreck A, Fritsch T, Huber W, Ringert RH, Jakse G, Fuzesi L (2003) Cytogenetic and morphologic typing of 58 papillary renal cell carcinomas: evidence for a cytogenetic evolution of type 2 from type 1 tumors. Cancer Res 63(19):6200–6205
Gunawan B, von Heydebreck A, Sander B, Schulten HJ, Haller F, Langer C, Armbrust T, Bollmann M, Gasparov S, Kovac D, Fuzesi L (2007) An oncogenetic tree model in gastrointestinal stromal tumours (GISTs) identifies different pathways of cytogenetic evolution with prognostic implications. J Pathol 211(4):463–470. doi:10.1002/path.2128
von Heydebreck A, Gunawan B, Fuzesi L (2004) Maximum likelihood estimation of oncogenetic tree models. Biostatistics 5(4):545–556. doi:10.1093/biostatistics/kxh007
Harada T, Okita K, Shiraishi K, Kusano N, Furuya T, Oga A, Kawauchi S, Kondoh S, Sasaki K (2002) Detection of genetic alterations in pancreatic cancers by comparative genomic hybridization coupled with tissue microdissection and degenerate oligonucleotide primed polymerase chain reaction. Oncology 62(3):251–258
Mahlamaki EH, Hoglund M, Gorunova L, Karhu R, Dawiskiba S, Andren-Sandberg A, Kallioniemi OP, Johansson B (1997) Comparative genomic hybridization reveals frequent gains of 20q, 8q, 11q, 12p, and 17q, and losses of 18q, 9p, and 15q in pancreatic cancer. Gene Chromosome Cancer 20(4):383–391
Schleger C, Arens N, Zentgraf H, Bleyl U, Verbeke C (2000) Identification of frequent chromosomal aberrations in ductal adenocarcinoma of the pancreas by comparative genomic hybridization (CGH). J Pathol 191(1):27–32. doi:10.1002/(sici)1096-9896(200005)191:1<27::aid-path582>3.0.co;2-j
Shiraishi K, Okita K, Kusano N, Harada T, Kondoh S, Okita S, Ryozawa S, Ohmura R, Noguchi T, Iida Y, Akiyama T, Oga A, Fukumoto Y, Furuya T, Kawauchi S, Sasaki K (2001) A comparison of DNA copy number changes detected by comparative genomic hybridization in malignancies of the liver, biliary tract and pancreas. Oncology 60(2):151–161
Fukushige S, Waldman FM, Kimura M, Abe T, Furukawa T, Sunamura M, Kobari M, Horii A (1997) Frequent gain of copy number on the long arm of chromosome 20 in human pancreatic adenocarcinoma. Genes Chromosomes Cancer 19(3):161–169
Zhao J, Moch H, Scheidweiler AF, Baer A, Schaffer AA, Speel EJ, Roth J, Heitz PU, Komminoth P (2001) Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer 32(4):364–372
Speel EJ, Richter J, Moch H, Egenter C, Saremaslani P, Rutimann K, Zhao J, Barghorn A, Roth J, Heitz PU, Komminoth P (1999) Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol 155(6):1787–1794
Stumpf E, Aalto Y, Hoog A, Kjellman M, Otonkoski T, Knuutila S, Andersson LC (2000) Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer 29(1):83–87. doi:10.1002/1098-2264(2000)9999:9999<::AID-GCC1011>3.0.CO;2-Z [pii]
Tonnies H, Toliat MR, Ramel C, Pape UF, Neitzel H, Berger W, Wiedenmann B (2001) Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut 48(4):536–541
Pourani J, Kaserer K, Pfragner R (2002) Cytogenetic and molecular analyses of multiple endocrine neoplasias of the MEN1 syndrome. Int J Oncol 20(5):971–976
Liu XP, Li DY, Liu XL, Xu JD, Furuya T, Kawauchi S, Oga A, Sasaki K (2009) Comparison of chromosomal aberrations between primary tumors and their synchronous lymph-node metastases in intestinal-type gastric carcinoma. Pathol Res Pract 205(2):105–111. doi:10.1016/j.prp.2008.09.003
Buffart TE, van Grieken NC, Tijssen M, Coffa J, Ylstra B, Grabsch HI, van de Velde CJ, Carvalho B, Meijer GA (2009) High resolution analysis of DNA copy-number aberrations of chromosomes 8, 13, and 20 in gastric cancers. Virchows Arch 455(3):213–223. doi:10.1007/s00428-009-0814-y
Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D, Ashkenazi A (1998) Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396(6712):699–703. doi:10.1038/25387
Aubele M, Auer G, Braselmann H, Nahrig J, Zitzelsberger H, Quintanilla-Martinez L, Smida J, Walch A, Hofler H, Werner M (2002) Chromosomal imbalances are associated with metastasis-free survival in breast cancer patients. Anal Cell Pathol 24(2–3):77–87
Kimura Y, Noguchi T, Kawahara K, Kashima K, Daa T, Yokoyama S (2004) Genetic alterations in 102 primary gastric cancers by comparative genomic hybridization: gain of 20q and loss of 18q are associated with tumor progression. Mod Pathol 17(11):1328–1337. doi:10.1038/modpathol.3800180
Kang JU, Kang JJ, Kwon KC, Park JW, Jeong TE, Noh SM, Koo SH (2006) Genetic alterations in primary gastric carcinomas correlated with clinicopathological variables by array comparative genomic hybridization. J Korean Med Sci 21(4):656–665
Goeze A, Schluns K, Wolf G, Thasler Z, Petersen S, Petersen I (2002) Chromosomal imbalances of primary and metastatic lung adenocarcinomas. J Pathol 196(1):8–16. doi:10.1002/path.1009
Wrage M, Ruosaari S, Eijk PP, Kaifi JT, Hollmen J, Yekebas EF, Izbicki JR, Brakenhoff RH, Streichert T, Riethdorf S, Glatzel M, Ylstra B, Pantel K, Wikman H (2009) Genomic profiles associated with early micrometastasis in lung cancer: relevance of 4q deletion. Clin Cancer Res 15(5):1566–1574. doi:10.1158/1078-0432.ccr-08-2188
Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2(12):897–909. doi:10.1038/nrc949
Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA (1997) Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386(6627):796–804. doi:10.1038/386796a0
Tarafa G, Villanueva A, Farre L, Rodriguez J, Musulen E, Reyes G, Seminago R, Olmedo E, Paules AB, Peinado MA, Bachs O, Capella G (2000) DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene 19(4):546–555. doi:10.1038/sj.onc.1203353
Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M (1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87(2):175–185
Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U (2002) c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol 15(4):462–469. doi:10.1038/modpathol.3880547
Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB (2002) Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 20(24):4673–4678
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765. doi:10.1056/NEJMoa0804385
Tsuchihashi Z, Khambata-Ford S, Hanna N, Janne PA (2005) Responsiveness to cetuximab without mutations in EGFR. N Engl J Med 353(2):208–209. doi:10.1056/NEJM200507143530218
Kulke MH, Hornick JL, Frauenhoffer C, Hooshmand S, Ryan DP, Enzinger PC, Meyerhardt JA, Clark JW, Stuart K, Fuchs CS, Redston MS (2009) O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 15(1):338–345. doi:10.1158/1078-0432.CCR-08-1476
Mayer P, Harjung A, Breinig M, Fischer L, Ehemann V, Malz M, Scherubl H, Britsch S, Werner J, Kern MA, Blaker H, Schirmacher P, Bergmann F (2012) Expression and therapeutic relevance of heat-shock protein 90 in pancreatic endocrine tumors. Endocr Relat Cancer 19(3):217–232. doi:10.1530/ERC-11-0227
Song D, Chaerkady R, Tan AC, Garcia-Garcia E, Nalli A, Suarez-Gauthier A, Lopez-Rios F, Zhang XF, Solomon A, Tong J, Read M, Fritz C, Jimeno A, Pandey A, Hidalgo M (2008) Antitumor activity and molecular effects of the novel heat shock protein 90 inhibitor, IPI-504, in pancreatic cancer. Mol Cancer Ther 7(10):3275–3284. doi:10.1158/1535-7163.MCT-08-0508
Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH, Sausville EA (2005) In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol 56(2):115–125. doi:10.1007/s00280-004-0939-2
Usmani SZ, Bona R, Li Z (2009) 17 AAG for HSP90 inhibition in cancer—from bench to bedside. Curr Mol Med 9(5):654–664
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549. doi:10.1038/nrc2887
Bergmann F, Wandschneider F, Sipos B, Moldenhauer G, Schniewind B, Welsch T, Schirrmacher P, Kloppel G, Altevogt P, Schafer H, Sebens Muerkoster S (2010) Elevated L1CAM expression in precursor lesions and primary and metastastic tissues of pancreatic ductal adenocarcinoma. Oncol Rep 24(4):909–915
Bergmann F, Moldenhauer G, Herpel E, Gaida MM, Strobel O, Werner J, Esposito I, Muerkoster SS, Schirmacher P, Kern MA (2010) Expression of L1CAM, COX-2, EGFR, c-KIT and Her2/neu in anaplastic pancreatic cancer: putative therapeutic targets? Histopathology 56(4):440–448. doi:10.1111/j.1365-2559.2010.03499.x
Kaifi JT, Zinnkann U, Yekebas EF, Schurr PG, Reichelt U, Wachowiak R, Fiegel HC, Petri S, Schachner M, Izbicki JR (2006) L1 is a potential marker for poorly-differentiated pancreatic neuroendocrine carcinoma. World J Gastroenterol 12(1):94–98
Gast D, Riedle S, Riedle S, Schabath H, Schlich S, Schneider A, Issa Y, Stoeck A, Fogel M, Joumaa S, Wenger T, Herr I, Gutwein P, Altevogt P (2005) L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int J Cancer 115(4):658–665. doi:10.1002/ijc.20869
Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze’ev A (2005) L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168(4):633–642. doi:10.1083/jcb.200408051
Kaifi JT, Strelow A, Schurr PG, Reichelt U, Yekebas EF, Wachowiak R, Quaas A, Strate T, Schaefer H, Sauter G, Schachner M, Izbicki JR (2006) L1 (CD171) is highly expressed in gastrointestinal stromal tumors. Mod Pathol 19(3):399–406. doi:10.1038/modpathol.3800547
Boo YJ, Park JM, Kim J, Chae YS, Min BW, Um JW, Moon HY (2007) L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol 14(5):1703–1711. doi:10.1245/s10434-006-9281-8
Fogel M, Mechtersheimer S, Huszar M, Smirnov A, Abu-Dahi A, Tilgen W, Reichrath J, Georg T, Altevogt P, Gutwein P (2003) L1 adhesion molecule (CD 171) in development and progression of human malignant melanoma. Cancer Lett 189(2):237–247
Issa Y, Nummer D, Seibel T, Muerkoster SS, Koch M, Schmitz-Winnenthal FH, Galindo L, Weitz J, Beckhove P, Altevogt P (2009) Enhanced L1CAM expression on pancreatic tumor endothelium mediates selective tumor cell transmigration. J Mol Med 87(1):99–112. doi:10.1007/s00109-008-0410-7
Gast D, Riedle S, Issa Y, Pfeifer M, Beckhove P, Sanderson MP, Arlt M, Moldenhauer G, Fogel M, Kruger A, Altevogt P (2008) The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene 27(9):1281–1289. doi:10.1038/sj.onc.1210747
Arlt MJ, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, Grunberg J, Honer M, Schubiger PA, Altevogt P, Kruger A (2006) Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res 66(2):936–943. doi:10.1158/0008-5472.CAN-05-1818
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516. doi:10.1056/NEJMoa1103782
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. doi:10.1056/NEJM200103153441101
Acknowledgments
The authors thank Regine Brandt, Maike Pacena, Anja Bredtmann, Andrea Müller, Stefanie Keller, and the NCT tissue bank Heidelberg for excellent technical support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 229 kb)
Rights and permissions
About this article
Cite this article
Bergmann, F., Aulmann, S., Sipos, B. et al. Acinar cell carcinomas of the pancreas: a molecular analysis in a series of 57 cases. Virchows Arch 465, 661–672 (2014). https://doi.org/10.1007/s00428-014-1657-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1657-8