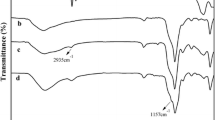
Abstract
High-substituted 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) was shown to self-assemble in aqueous solutions in the presence of in situ-formed insoluble species of iron. Two different types of water-soluble high-molecular compounds were synthesized at room temperature in aqueous aerated alkaline solutions of (NH4)2Fe(SO4)2•6H2O and high-substituted 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) in the presence of NaH2PO2. These substances called adducts proved to be inorganic-organic hybrid nanospecies of iron species, HP-β-CD and encapsulated inorganic salts (sulfates and phosphates of sodium and ammonium). The adducts were established to show different properties and to have diverse compositions depending on the HP-β-CD: Fe molar ratio in starting solution. The first synthesized adduct (I) is characterized by an unambiguous magnetism with lower solubility in water. It gives a homogeneous solution with particles 400 nm in diameter. The second adduct (II) is not magnetic, more soluble in water, and forms nanoparticles of ∼760 nm in diameter. Both adducts are stable in aqueous solutions but totally destroyed in the presence of H2O2, K2S2O8, and AgNO3. By addition of BaCl2 excess to the aqueous solutions of I and II, the residue of BaSO4 is precipitated and dimensions of the particles of both types decrease up to ∼230 and ∼570 nm in diameter, respectively. Solids I and II were characterized by the data of X-ray diffraction (XRD), Mössbauer spectroscopy, SEM, IR-spectra, and magnetic measurements. Aqueous solutions of I and II were studied by means of mass-spectrometry (MALDI-TOF), dynamic light scattering (DLS) measurements, and analyzed by spectrophotometric titration with phenolphthalein.











Similar content being viewed by others
Notes
Authors are appreciated to S.M. Khomutov for 2-hydroxypropyl-β-cyclodextrin samples
Abbreviations
- HP − β − CD:
-
2-Hydroxypropyl-β-cyclodextrin
- MALDI-TOF:
-
Matrix assisted laser desorption/ionization time of flight
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- IR-spectra:
-
Infrared spectra
- DLS:
-
Dynamic light scattering
References
Boal AK, Ilhan F, DeRouchey JE, Thurn-Albrecht T, Russell TP, Rotello VM (2000) Self-assembly of nanoparticles into structured spherical and network aggregates. Lett Nat 404:746–748. doi:10.1038/35008037
Sanchez C, Lebeau B (2001) Design and properties of hybrid organic-inorganic nanocomposites for photonics mater. Res Soc Bull 6:377–387
Whitesides GM, Mathias JP, Seto C (1991) Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science 254:1312–1319. doi:10.1126/science.1962191
Sanchez C, Beatriz J, Bellevill P, Popall M (2005) Applications of hybrid organic-inorganic nanocomposites. J Mater Chem 15:3559–3592. doi:10.1039/b509097k
Jing B, Chen X, Wang X, Yurong Z, Qiu H (2008) Sol-gel-sol transition of gold nanoparticle-based supramolecular hydrogels induced by cyclodextrin inclusion. ChemPhysChem 9(2):249–252. doi:10.1002/cphc.200700625
Wang Y, Wong JF, Teng X, Lin XZ, Yang H (2003) “Pulling” nanoparticles into water: phase transfer of oleic acid stabilized monodisperse nanoparticles into aqueous solutions of α-cyclodextrin. Nano Lett 3(11):1555–1559. doi:10.1021/nl034731j
Zhou S, Wang L, Zhang A, Lin K, Liu W (2008) Preparation, stabilization, and bioefficacy of beta-cyclodextrin inclusion compounds of chloramidophos. J Agric Food Chem 56:2708–2713. doi:10.1021/jf703635
Gao Y, Zhao X, Dong B, Zheng L, Li N, Zhang S (2006) Inclusion complexes of beta-cyclodextrin with ionic liquid surfactants. J Phys Chem 110:8576–8581
Fernandes JA, Lima S, Braga SS, Pillinger M, Ribeiro-Claro P, Rodrigez-Borges JE, Lopes AD, Teixeira-Dias JJC, Goncüalves IS (2005) Interactions of cationic and neutral molybdenum complexes with β-cyclodextrin host molecules. Organometallics 24:5673–5677. doi:10.1021/om001088s
Hubert C, Denicourt-Nowicki I, Roucoux A, Landy D, Leger B, Crowyn G, Monflier E (2009) Catalytically active nanoparticles stabilized by host-guest inclusion complexes in water. Chem Commun 1:1228–1230. doi:10.1039/B818786J
Nowicki A, Zhang Y, Léger B, Rolland JP, Bricout H, Monflier E, Roucoux A (2006) Supramolecular shuttle and protective agent: a multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium nanoparticles. Chem Commun 296:924–945. doi:10.1039/B512838B
He SH, Shi W, Zhang X, Li J, Huang Y (2010) β-cyclodextrins-based inclusion complexes of CoFe2O4 magnetic nanoparticles as catalyst for the luminol chemiluminescence system and their applications in hydrogen peroxide detection. Talanta 82:377–383
Chung JW, Kwak S-Y (2010) Iron-induced cyclodextrin self-assembly into size-controllable nanospheres. Langmuir 26(4):2418–2423. doi:10.1021/la9028645
Yang C, Cheung YK, Yao J, Wong YJ, Jia G (2001) Palladium and platinum complexes with a β-cyclodextrin-functionalized phosphine ligand. Organometallics 20(3):424–429. doi:10.1021/om000634e
Zhang H, Peng M-L, Cui Y-L, Chen C (2008) Magnetic hp-β-cd composite nanoparticle: synthesis, characterization and application as a carrier of doxorubicin in vitro. Chin J Chem 26:1737–1740. doi:10.1002/cjoc.200890314
Topchieva IN, Spiridonov VV, Kataeva NA, Gubin SP, Filippov SK, Lezov AV (2006) Magnetic nanocomposites based on cyclodextrin-containing molecular tubes and iron nanoparticles. Colloid Polim Sci 284:795–801. doi:10.1007/s00396-005-1456-5
Spiridonov VV, Topchieva IN, Zakharov AN, Afanasov MI, Perov NS (2009) Method of preparation of water-soluble adducts based on magneto-ordered iron oxides and substituted β-cyclodextrins RF 2453499 10 dec. (Patent)
Wu Y, Zuo F, Zheng Z, Ding X, Peng Y (2009) A novel approach to molecular recognition surface of magnetic nanoparticles based on host-guest effect. Nanoscale Res Lett 4:738–747. doi:10.1007/s11671-009-9314-x
Sueishi Y, Miyakawa T (1999) Complexation of phenols with β- and γ-cyclodextrins: determination of the association constants by using the isomerization of spiropyran. J Phys Org Chem 12:541–546. doi:10.1002/(SICI)1099-1395(199907)12:7
Mäkelä M, Korpela T, Laakso SJ (1987) Colorimetric determination of beta-cyclodextrin: two assay modifications based on molecular complexation of phenolphthalein. J Biochem Biophys Methods 14:85–92
Sasaki KJ, Christian CSD, Tucker EE (1989) Study of the stability of 1:1 complexes between aliphatic alcohols and β-cyclodextrins in aqueous solution. Fluid Phase Equilib 49:281–289. doi:10.1016/0378-3812(89)80022-6
Conflicts of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
The angular dependence of the effective (apparent) radius (R h ) of adduct I. С(adduct I) = 1 % wt.; Т = 298 K; Accumulation time of ACF: t = 5 min
ESM 2
The angular dependence of the effective (apparent) radius (R h ) of adduct II. С(adduct II) = 1 % wt.; Т = 298 K; accumulation time of ACF: t = 5 min
ESM 3
MALDI-spectrum of HP-β-CD (DS = 6.8)
ESM 4
MALDI-spectrum of the adduct I obtained from HP-β-CD (DS = 6.8)
ESM 5
MALDI-spectrum of the adduct II synthesized from HP-β-CD (DS = 6.8)
ESM 6
Scanning electron microscopy images of dried adduct I HP-β-CD with magnetite obtained from the solution during lyophilization (A); dried samples of HP-β-СD and Na2SO4 received in the similar way (B and C, respectively)
ESM 7
A The autocorrelation functions (ACF) of light scattering by solution of adduct I at Θ = 80°, 90°, 100°; B Distribution function of adduct I particles by the effective hydrodynamic radii (R h ) at Θ = 90°; С(adduct I) = 1 % wt.; Т = 298 K; accumulation time of ACF: t = 5 min
ESM 8
A The autocorrelation functions (ACF) of light scattering by solution of adduct II at Θ = 80°, 90°, 100°; B Distribution function of adduct II particles by the effective hydrodynamic radii (R h ) at Θ = 90°; С(adduct II) = 1 % wt.; Т = 298 K; accumulation time of ACF: t = 5 min
ESM 9
Plots of effective hydrodynamic radii of the species of the type I (1) and II (2) vs. their concentrations (mass%) in aqueous solutions at 25 °C; θ = 90°
Rights and permissions
About this article
Cite this article
Spiridonov, V.V., Zakharov, A.N., Panova, I.G. et al. Self-assembling of highly substituted 2-hydroxypropyl-β-cyclodextrin in the presence of in situ-formed iron oxide nanoparticles to produce magnetically ordered water-soluble supramolecular adducts. Colloid Polym Sci 293, 1329–1337 (2015). https://doi.org/10.1007/s00396-015-3514-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3514-y