Abstract
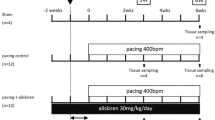
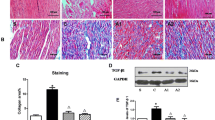
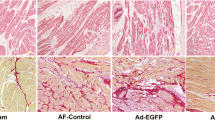
Effects of an angiotensin II receptor blocker, irbesartan (IRB), on the development of atrial fibrosis and atrial fibrillation (AF) were assessed in a canine model of atrial tachycardia remodeling (ATR) with left ventricular dysfunction, together with its possible association with involvement of p53. Atrial tachypacing (400 bpm for 4 weeks) was used to induce ATR in beagles treated with placebo (ATR-dogs, n = 6) or irbesartan (IRB-dogs, n = 5). Non-paced sham dogs served as control (Control-dogs, n = 4). ATR- and IRB-dogs developed tachycardia-induced left ventricular dysfunction. Atrial effective refractory period (AERP) shortened (83 ± 5 ms, p < 0.05), inter-atrial conduction time prolonged (72 ± 2 ms, p < 0.05), and AF duration increased (29 ± 5 s, p < 0.05 vs. baseline) after 4 weeks in ATR-dogs. ATR-dogs also had a larger area of atrial fibrous tissue (5.2 ± 0.5 %, p < 0.05 vs. Control). All these changes, except for AERP, were attenuated in IRB-dogs (92 ± 3 ms, 56 ± 3 ms, 9 ± 5 s, and 2.5 ± 0.7 %, respectively; p < 0.05 vs. ATR for each). In ATR-dogs, p53 expression in the left atrium decreased by 42 % compared with Control-dogs (p < 0.05); however, it was highly expressed in IRB-dogs (+89 % vs. ATR). Transforming growth factor (TGF)-β1 expression was enhanced in ATR-dogs (p < 0.05 vs. Control) but reduced in IRB-dogs (p < 0.05 vs. ATR). Irbesartan suppresses atrial fibrosis and AF development in a canine ATR model with left ventricular dysfunction in association with p53.






Similar content being viewed by others
References
Iwasaki YK, Nishida K, Kato T, Nattel S (2011) Atrial fibrillation pathophysiology: implications for management. Circulation 124:2264–2274
Nishida K, Nattel S (2014) Atrial fibrillation compendium: historical context and detailed translational perspective on an important clinical problem. Circ Res 114:1447–1452
Kato K, Ejima K, Fukushima N, Ishizawa M, Wakisaka O, Henmi R, Yoshida K, Nuki T, Arai K, Yashiro B, Manaka T, Ashihara K, Shoda M, Hagiwara N (2016) Catheter ablation of atrial fibrillation in patients with severely impaired left ventricular systolic function. Heart Vessels 31:584–592
Burstein B, Libby E, Calderone A, Nattel S (2008) Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 117:1630–1641
Nishida K, Michael G, Dobrev D, Nattel S (2010) Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 12:160–172
Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S (2007) Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc Res 76:442–452
Ehrlich JR, Hohnloser SH, Nattel S (2006) Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J 5:512–518
Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S (2001) Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 104:2608–2614
Sakabe M, Fujiki A, Nishida K, Sugao M, Nagasawa H, Tsuneda T, Mizumaki K, Inoue H (2004) Enalapril prevents perpetuation of atrial fibrillation by suppressing atrial fibrosis and over-expression of connexin43 in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Pharmacol 43:851–859
Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K (2000) Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 101:2612–2617
Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K (2003) Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol 41:2197–2204
Ghosh AK, Bhattacharyya S, Varga J (2004) The tumor suppressor p53 abrogates Smad-dependent collagen gene induction in mesenchymal cells. J Biol Chem 279:47455–47463
Zhang Y, Köhler K, Xu J, Lu D, Braun T, Schlitt A, Buerke M, Müller-Werdan K, Ebelt H (2011) Inhibition of p53 after acute myocardial infarction: reduction of apoptosis is counteracted by disturbed scar formation and cardiac rupture. J Mol Cell Cardiol 50:471–478
Tamaki Y, Iwanaga Y, Niizuma S, Kawashima T, Kato T, Inuzuka Y, Horie T, Morooka H, Takase T, Akahashi Y, Kobuke K, Ono K, Shioi T, Sheikh SP, Ambartsumian N, Lukanidin E, Koshimizu TA, Miyazaki S, Kimura T (2013) Metastasis-associated protein, S100A4 mediates cardiac fibrosis potentially through the modulation of p53 in cardiac fibroblasts. J Mol Cell Cardiol 57:72–81
Zhu F, Li Y, Zhang J, Piao C, Liu T, Li HH, Du J (2013) Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One 8:e74535
Madrid AH, Marin IM, Cervantes CE, Morell EB, Estévez JE, Moreno G, Parajón JR, Peng J, Limón L, Nannini S, Moro C (2004) Prevention of recurrences in patients with lone atrial fibrillation. The dose-dependent effect of angiotensin II receptor blockers. J Renin Angiotensin Aldosterone Syst 5:114–120
Perry GJ, Wei CC, Hankes GH, Dillon SR, Rynders P, Mukherjee R, Spinale FG, Dell’Italia LJ (2002) Angiotensin II receptor blockade does not improve left ventricular function and remodeling in subacute mitral regurgitation in the dog. J Am Coll Cardiol 39:1374–1379
Huang XH, Qiu FR, Xie HT, Li J (2005) Pharmacokinetic and pharmacodynamic interaction between irbesartan and hydrochlorothiazide in renal hypertensive dogs. J Cardiovasc Pharmacol 46:863–869
Nishida K, Sarrazin JF, Fujiki A, Oral H, Inoue H, Morady F, Nattel S (2010) Roles of the left atrial roof and pulmonary veins in the anatomic substrate for persistent atrial fibrillation and ablation in a canine model. J Am Coll Cardiol 56:1728–1736
Nakatani Y, Nishida K, Sakabe M, Kataoka N, Sakamoto T, Yamaguchi Y, Iwamoto J, Mizumaki K, Fujiki A, Inoue H (2013) Tranilast prevents atrial remodeling and development of atrial fibrillation in a canine model of atrial tachycardia and left ventricular dysfunction. J Am Coll Cardiol 61:582–588
Hanna N, Cardin S, Leung TK, Nattel S (2004) Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res 63:236–244
Verheule S, Sato T, Everett T 4th, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE (2004) Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res 94:1458–1465
Nakajima H, Nakajima HO, Salcher O, Dittlé AS, Dembowsky K, Jing S, Field LJ (2000) Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-β1 transgene in the heart. Circ Res 86:571–579
Takii E, Inage T, Yoshida T, Ohe M, Gondo T, Haraguchi G, Ito S, Kumanomido J, Imaizumi T, Fukuomoto Y (2016) Beneficial effects of losartan for prevention of paroxysmal atrial fibrillation in patients with sick sinus syndrome: analysis with memory function of pacemaker. Heart Vessels 31:402–407
Chilukoti RK, Mostertz J, Bukowska A, Aderkast C, Felix SB, Busch M, Völker U, Goette A, Wolke C, Homuth G, Lendeckel U (2013) Effects of irbesartan on gene expression revealed by transcriptome analysis of left atrial tissue in a porcine model of acute rapid pacing in vivo. Int J Cardiol 168:2100–2108
Zhang ZZ, Shang QH, Jin HY, Song B, Oudit GY, Lu L, Zhou T, Xu YL, Gao PJ, Zhu DL, Penninger JM, Zhong JC (2013) Cardiac protective effects of irbesartan via the PPAR-gamma signaling pathway in angiotensin-converting enzyme 2-deficient mice. J Transl Med 11:229
Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8:275–283
Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89:279–286
Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G (2007) Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation 115:2925–2930
Acknowledgments
The authors thank Sanofi K. K. and Shionogi & Co., Ltd. for supplying irbesartan. The authors wish to thank Kiyomi Note for her expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by research funding from Shionogi & Co., Ltd. and JSPS KAKENHI Grant Number 24591039.
Rights and permissions
About this article
Cite this article
Kataoka, N., Nishida, K., Kinoshita, K. et al. Effect of irbesartan on development of atrial fibrosis and atrial fibrillation in a canine atrial tachycardia model with left ventricular dysfunction, association with p53. Heart Vessels 31, 2053–2060 (2016). https://doi.org/10.1007/s00380-016-0853-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0853-7