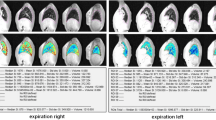
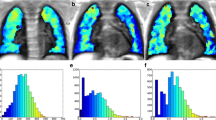
Abstract
Objectives
To explore the feasibility of diffusion-weighted imaging (DWI) to assess inflammatory lung changes in patients with Cystic Fibrosis (CF)
Methods
CF patients referred for their annual check-up had spirometry, chest-CT and MRI on the same day. MRI was performed in a 1.5 T scanner with BLADE and EPI-DWI sequences (b = 0–600 s/mm2). End-inspiratory and end-expiratory scans were acquired in multi-row scanners. DWI was scored with an established semi-quantitative scoring system. DWI score was correlated to CT sub-scores for bronchiectasis (CF-CTBE), mucus (CF-CTmucus), total score (CF-CTtotal-score), FEV1, and BMI. T-test was used to assess differences between patients with and without DWI-hotspots.
Results
Thirty-three CF patients were enrolled (mean 21 years, range 6–51, 19 female). 4 % (SD 2.6, range 1.5-12.9) of total CF-CT alterations presented DWI-hotspots. DWI-hotspots coincided with mucus plugging (60 %), consolidation (30 %) and bronchiectasis (10 %). DWItotal-score correlated (all p < 0.0001) positively to CF-CTBE (r = 0.757), CF-CTmucus (r = 0.759) and CF-CTtotal-score (r = 0.79); and negatively to FEV1 (r = 0.688). FEV1 was significantly higher (p < 0.0001) in patients without DWI-hotspots.
Conclusions
DWI-hotspots strongly correlated with radiological and clinical parameters of lung disease severity. Future validation studies are needed to establish the exact nature of DWI-hotspots in CF patients.
Key Points
• DWI hotspots only partly overlapped structural abnormalities on morphological imaging
• DWI strongly correlated with radiological and clinical indicators of CF-disease severity
• Patients with more DWI hotspots had lower lung function values
• Mucus score best predicted the presence of DWI-hotspots with restricted diffusion.





Similar content being viewed by others
References
Tiddens HAWM, Donaldson SH, Rosenfeld M, Paré PD (2010) Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol 45:107–117
Loeve M, Gerbrands K, Hop WC et al (2011) Bronchiectasis and pulmonary exacerbations in children and young adults with cystic fibrosis. Chest 140:178–185
Conway S, Balfour-Lynn IM, De Rijcke K et al (2014) European cystic fibrosis society standards of care: framework for the cystic fibrosis centre. J Cyst Fibros 13:S3–S22
Grasemann H, Ratjen F (2013) Early lung disease in cystic fibrosis. Lancet Respir Med 1:148–157
Eichinger M, Heussel C-P, Kauczor H-U et al (2010) Computed tomography and magnetic resonance imaging in cystic fibrosis lung disease. J Magn Reson Imaging 32:1370–1378
Loeve M, Hop WCJ, De Bruijne M et al (2012) Chest computed tomography scores are predictive of survival in patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med 185:1096–1103
Kuo W, Ciet P, Tiddens HAWM et al (2014) Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med 189:1328–1336
Wielpütz MO, Eichinger M, Puderbach M (2013) Magnetic resonance imaging of cystic fibrosis lung disease. J Thorac Imaging 28:151–159
Failo R, Wielopolski PA, Tiddens HAWM et al (2009) Lung morphology assessment using MRI: a robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients. Magn Reson Med 61:299–306
Ciet P, Serra G, Bertolo S et al (2015) Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol. doi:10.1007/s00330-015-3850-9
Ma W, Sheikh K, Svenningsen S et al (2015) Ultra-short echo-time pulmonary MRI: Evaluation and reproducibility in COPD subjects with and without bronchiectasis. J Magn Reson Imaging 41:1465–1474
Dournes G, Grodzki D, Macey J et al (2015) Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology 276:258–265
Tiddens HAWM, Stick SM, Wild JM et al (2015) Respiratory tract exacerbations revisited: ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment. Pediatr Pulmonol 50:S57–S65
Bauman G, Puderbach M, Heimann T et al (2013) Validation of fourier decomposition MRI with dynamic contrast-enhanced MRI using visual and automated scoring of pulmonary perfusion in young cystic fibrosis patients. Eur J Radiol 82:2371–2377
van Beek EJR, Hill C, Woodhouse N et al (2007) Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-Helium MRI: comparison with shwachman score, chrispin-Norman score and spirometry. Eur Radiol 17:1018–1024
Stadler A, Stiebellehner L, Jakob PM et al (2007) Quantitative and o(2) enhanced MRI of the pathologic lung: findings in emphysema, fibrosis, and cystic fibrosis. Int J Biomed Imaging 2007:23624
Ciet P, Tiddens HAWM, Wielopolski PA et al (2015) Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol. doi:10.1007/s00247-015-3420-y
Attariwala R, Picker W (2013) Whole body MRI: improved lesion detection and characterization with diffusion weighted techniques. J Magn Reson Imaging 38:253–268
Luna A, Sánchez-Gonzalez J, Caro P (2011) Diffusion-weighted imaging of the chest. Magn Reson Imaging Clin N Am 19:69–94
Gaviani P, Schwartz RB, Hedley-Whyte ET et al (2005) Diffusion-weighted imaging of fungal cerebral infection. Am J Neuroradiol 26:1115–1121
Moritani T, Kim J, Capizzano AA et al (2014) Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol 87:20140011
Qi J, Olsen NJ, Price RR et al (2008) Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J Magn Reson Imaging 27:212–217
Ream JM, Dillman JR, Adler J et al (2013) MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol 43:1077–1085
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338
Tepper LA, Ciet P, Caudri D et al (2015) Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr Pulmonol. doi:10.1002/ppul.23328
Tepper LA, Utens EMWJ, Caudri D et al (2013) Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur Respir J 42:371–379
Liu H, Liu Y, Yu T, Ye N (2010) Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur Radiol 20:807–815
Tanaka R, Horikoshi H, Nakazato Y et al (2009) Magnetic resonance imaging in peripheral lung adenocarcinoma: correlation with histopathologic features. J Thorac Imaging 24:4–9
Uto T, Takehara Y, Nakamura Y et al (2009) Higher sensitivity and specificity for diffusion-weighted imaging of malignant lung lesions without apparent diffusion coefficient quantification. Radiology 252:247–254
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2 edn. Routledge
Geert V, Molenberghs G (2000) Linear mixed models for longitudinal data, 1st edn. doi:10.1007/978-1-4419-0300
Burnham KP (2004) Multimodel Inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304
Deng Y, Li X, Lei Y, Liang C, Liu Z (2015) Use of diffusion-weighted magnetic resonance imaging to distinguish between lung cancer and focal inflammatory lesions: a comparison of intravoxel incoherent motion derived parameters and apparent diffusion coefficient. Acta Radiol 1–8. doi:10.1177/0284185115586091
Le Bihan D (2008) Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology 249:748–752
Chavhan GB, Alsabban Z, Babyn PS (2014) Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics 34:E73–E88
Acknowledgments
The authors thank Elizabeth Salamon, Department of Respiratory Medicine, Royal Perth Hospital, for offering invaluable detailed advice on grammar and organization of the manuscript. The authors also thank Thorsten Feiweier, Siemens Healthcare Erlangen, Germany, for technical advice in critically reviewing the manuscript. The researchers also wish to express their deepest gratitude to all CF patients, who participated in the study.
The scientific guarantor of this publication is Dr. Giovanni Morana, chief of the Radiology Department at Ca’Foncello General Hospital, Treviso, Italy. The authors of this manuscript declare relationships with the following companies: Bracco Imaging (not related to this article). The authors of this manuscript declare no other relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by the Italian cystic fibrosis league (Lega Italiana Fibrosi Cistica, LIFC) to cover the travel expenses of the first author.
Eleni Rosalina Andrinopoulou, statistician, kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained in all participating centres. Written informed consent was obtained from all subjects (patients) in this study. Study subjects have been previously reported in Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. European Radiology DOI: 10.1007/s00330-015-3850-9 . Methodology: prospective, cross sectional study, observational, multicenter study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online supplement digital content 1
DWI scoring sheet. DWI signal is assessed in highest b-value images (b = 600 s/mm2). Signal intensity (SI) of DWI hotspots is compared to SI of the spine and scored 1 when SI hotspots is lower than SI spine, or scored 2 when SI hotspot is equal or greater of SI spine. The number of hotspots is counted per lobe, with the lingula considered a separate lobe. Moreover, each hotspot is identified in the same categories defined in the CF-CT and CF-MRI scoring system. RUL= Right upper lobe, RML=Right middle lobe, RLL=Right lower lobe, LUL=Left upper lobe, Lin=Lingula, LLL=Left lower lobe (JPG 8 kb)
Online supplement digital content 2
(DOCX 17 kb)
Online supplement digital content 3
A) Bland-Altman for total number of DWI hotspots identified by observer 1 (Obs1) and observer 2 (Obs2). Thick line is the mean, dashed lines are ± 2 standard deviations (SD). B) Identity Plot total DWI hotspots Obs1 versus Obs2. The continuous line is the identity line (y = 1*x + 0), dashed line is the correlations line. Note that that Obs2 detected more hotspots than Obs1 (mean difference ~ 3). (JPG 3 kb)
(JPG 3 kb)
Rights and permissions
About this article
Cite this article
Ciet, P., Serra, G., Andrinopoulou, E.R. et al. Diffusion weighted imaging in cystic fibrosis disease: beyond morphological imaging. Eur Radiol 26, 3830–3839 (2016). https://doi.org/10.1007/s00330-016-4248-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4248-z