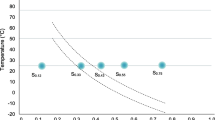
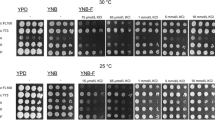
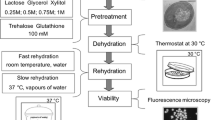
Abstract
Yeast cells are well adapted to interfacial habitats, such as the surfaces of soil or plants, where they can resist frequent fluctuations between wet and dry conditions. Saccharomyces cerevisiae is recognized as an anhydrobiotic organism, and it has been the subject of numerous studies that aimed to elucidate this ability. Extensive data have been obtained from these studies based on a wide range of experimental approaches, which have added significantly to our understanding of the cellular bases and mechanisms of resistance to desiccation. The aim of this review is to provide an integrated view of these mechanisms in yeast and to describe the survival kit of S. cerevisiae for anhydrobiosis. This kit comprises constitutive and inducible mechanisms that prevent cell damage during dehydration and rehydration. This review also aims to characterize clearly the phenomenon of anhydrobiosis itself based on detailed descriptions of the causes and effects of the constraints imposed on cells by desiccation. These constraints mainly lead to mechanical, structural, and oxidative damage to cell components. Considerations of these constraints and the possible utilization of components of the survival kit could help to improve the survival of sensitive cells of interest during desiccation.

Similar content being viewed by others
References
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111:1–14
Babazadeh R, Adiels CB, Smedh M, Petelenz-Kurdziel E, Goksor M, Hohmann S (2013) Osmostress-induced cell volume loss delays yeast Hog1 signaling by limiting diffusion processes and by Hog1-specific effects. PLoS One 8(11):e80901
Bailly C, Leymarie J, Lehner A, Rousseau S, Côme D, Corbineau F (2004) Catalase activity and expression in developing sunflower seeds as related to drying. J Exp Bot 55(396):475–483
Ball P (2008) Water as an active constituent in cell biology. Chem Rev 108(1):74–108
Beker M, Blumbergs J, Ventina E, Rapoport A (1984) Characteristics of cellular membranes at rehydration of dehydrated yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 19(5):347–352
Beker MJ, Rapoport AI (1987) Conservation of yeasts by dehydration biotechnology methods. Springer, pp 127-171
Benaroudj N, Lee DH, Goldberg AL (2001) Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem 276(26):24261–24267
Beney L, Gervais P (2001) Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl Microbiol Biotechnol 57(1–2):34–42
Beney L, Linares E, Ferret E, Gervais P (1998) Influence of the shape of phospholipid vesicles on the measurement of their size by photon correlation spectroscopy. Eur Biophys J 27(6):567–574
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272(33):20313–20316
Billi D, Wright DJ, Helm RF, Prickett T, Potts M, Crowe JH (2000) Engineering desiccation tolerance in Escherichia coli. Appl Environ Microbiol 66(4):1680–1684
Biryuzova VI, Rapoport AI (1978) Cryofractographic studies of yeast cell structures in anabiosis. Microbiology 47(2):245–251
Blagoeva J, Stoev G, Venkov P (1991) Glucan structure in a fragile mutant of Saccharomyces cerevisiae. Yeast 7(5):455–461. doi:10.1002/yea.320070504
Bottema CD, McLean-Bowen CA, Parks LW (1983) Role of sterol structure in the thermotropic behavior of plasma membranes of Saccharomyces cerevisiae. Biochim Biophys Acta 734(2):235–248
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. Bioessays 28(8):799–808. doi:10.1002/bies.20441
Brovchenko I, Krukau A, Oleinikova A, Mazur AK (2007) Water clustering and percolation in low hydration DNA shells. J Phys Chem B 111(12):3258–3266
Brown AD (1976) Microbial water stress. Bacteriol Rev 40(4):803–846
Calahan D, Dunham M, DeSevo C, Koshland DE (2011) Genetic analysis of desiccation tolerance in Saccharomyces cerevisiae. Genetics 189(2):507–519
Carpenter JF, Crowe JH (1989) An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry (Mosc) 28(9):3916–3922
Catalá A (2009) Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 157(1):1–11
Cerrutti P, de Huergo MS, Galvagno M, Schebor C, del Pilar Buera M (2000) Commercial baker's yeast stability as affected by intracellular content of trehalose, dehydration procedure and the physical properties of external matrices. Appl Microbiol Biotechnol 54(4):575–580
Cheung MS, Klimov D, Thirumalai D (2005) Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci U S A 102(13):4753–4758
Clegg JS, Seitz P, Seitz W, Hazlewood CF (1982) Cellular responses to extreme water loss: the water-replacement hypothesis. Cryobiology 19(3):306–316
Cooper GM, Hausman RE (2000) The cell. Sinauer Associates Sunderland
Costa V, Reis E, Quintanilha A, Moradasferreira P (1993) Acquisition of ethanol tolerance in Saccharomyces cerevisiae: the key role of the mitochondrial superoxide dismutase. Arch Biochem Biophys 300(2):608–614
Crowe JH, Crowe LM, Chapman D (1984) Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223(4637):701–703
Crowe JH, Crowe LM, Hoekstra FA (1989) Phase transitions and permeability changes in dry membranes during rehydration. J Bioenerg Biomembr 21(1):77–91
Crowe JH, Hoekstra FA, Crowe LM (1992) Anhydrobiosis. Annu Rev Physiol 54(1):579–599
Crowe JH, Panek AD, Crowe LM, Panek A, De Araujo PS (1991) Trehalose transport in yeast cells. Biochem Int 24(4):721–730
de Jesus Pereira E, Panek AD, Eleutherio ECA (2003) Protection against oxidation during dehydration of yeast. Cell Stress Chaperones 8(2):120
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233:351–371
Despa F (2006) Biological water. Ann N Y Acad Sci 1066(1):1–11
Dupont S, Beney L, Ferreira T, Gervais P (2011) Nature of sterols affects plasma membrane behavior and yeast survival during dehydration. Biochim Biophys Acta 1808(6):1520–1528
Dupont S, Beney L, Ritt JF, Lherminier J, Gervais P (2010) Lateral reorganization of plasma membrane is involved in the yeast resistance to severe dehydration. Biochim Biophys Acta 1798(5):975–985
Dupont S, Lemetais G, Ferreira T, Cayot P, Gervais P, Beney L (2012) Ergosterol biosynthesis: a fungal pathway for life on land? Evolution 66(9):2961–2968
Eggers DK, Valentine JS (2001) Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci 10(2):250–261
Eleutherio EC, de Araujo PS, Panek AD (1993) Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim Biophys Acta 1156(3):263–266
Espindola AS, Gomes DS, Panek AD, Eleutherio ECA (2003) The role of glutathione in yeast dehydration tolerance. Cryobiology 47(3):236–241
Evans EA, Waugh R, Melnik L (1976) Elastic area compressibility modulus of red cell membrane. Biophys J 16(6):585–595
Fernández Murga ML, Bernik D, Font de Valdez G, Disalvo AE (1999) Permeability and stability properties of membranes formed by lipids extracted from Lactobacillus acidophilus grown at different temperatures. Arch Biochem Biophys 364(1):115–121
Ferrigno P, Posas F, Koepp D, Saito H, Silver PA (1998) Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J 17(19):5606–5614
Fraikin GY, Strakhovskaya M, Rubin A (1996) The role of membrane-bound porphyrin-type compound as endogenous sensitizer in photodynamic damage to yeast plasma membranes. J Photochem Photobiol B 34(2):129–135
França M, Panek A, Eleutherio E (2007) Oxidative stress and its effects during dehydration. Comp Biochem Phys A 146(4):621–631
França MB, Panek AD, Eleutherio EC (2005) The role of cytoplasmic catalase in dehydration tolerance of Saccharomyces cerevisiae. Cell Stress Chaperones 10(3):167–170
Fuller W, Forsyth T, Mahendrasingam A (2004) Water–DNA interactions as studied by X-ray and neutron fibre diffraction. Philos Trans R Soc Lond B Biol Sci 359(1448):1237–1248
Gadd G, Chalmers K, Reed R (1987) The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett 48(1):249–254
Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, Covarrubias AA (2000) Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J Biol Chem 275(8):5668–5674
Garner MM, Burg MB (1994) Macromolecular crowding and confinement in cells exposed to hypertonicity. Am J Physiol Cell Physiol 266(4):C877–C892
Garre E, Raginel F, Palacios A, Julien A, Matallana E (2010) Oxidative stress responses and lipid peroxidation damage are induced during dehydration in the production of dry active wine yeasts. Int J Food Microbiol 136(3):295–303
Gechev TS, Dinakar C, Benina M, Toneva V, Bartels D (2012) Molecular mechanisms of desiccation tolerance in resurrection plants. Cell Mol Life Sci 69(19):3175–3186. doi:10.1007/s00018-012-1088-0
Gervais P, Beney L (2001) Osmotic mass transfer in the yeast Saccharomyces cerevisiae. Cell Mol Biol (Noisy-le-grand) 47(5):831–839
Golovina E, Golovin A, Hoekstra F, Faller R (2010) Water replacement hypothesis in atomic details: effect of trehalose on the structure of single dehydrated POPC bilayers. Langmuir 26(13):11118–11126
Golovina EA, Golovin AV, Hoekstra FA, Faller R (2009) Water replacement hypothesis in atomic detail—factors determining the structure of dehydrated bilayer stacks. Biophys J 97(2):490–499
Gómez-Pastor R, Pérez-Torrado R, Cabiscol E, Ros J, Matallana E (2010) Reduction of oxidative cellular damage by overexpression of the thioredoxin TRX2 gene improves yield and quality of wine yeast dry active biomass. Microb Cell Factories 9(9):10.1186
Goyal K, Walton L, Tunnacliffe A (2005a) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157
Goyal K, Walton LJ, Browne JA, Burnell AM, Tunnacliffe A (2005b) Molecular anhydrobiology: identifying molecules implicated in invertebrate anhydrobiosis. Integr Comp Biol 45(5):702–709
Guzhova I, Krallish I, Khroustalyova G, Margulis B, Rapoport A (2008) Dehydration of yeast: changes in the intracellular content of Hsp70 family proteins. Process Biochem 43(10):1138–1141
Halliwell B (1987) Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem Phys Lipids 44(2):327–340
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57(5):715S–724S
Hays LM, Crowe JH, Wolkers W, Rudenko S (2001) Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology 42(2):88–102
Herdeiro R, Pereira M, Panek A, Eleutherio E (2006) Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim Biophys Acta 1760(3):340–346
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6(9):431–438
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66(2):300–372
Howlett NG, Avery SV (1997) Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol 63(8):2971–2976
Israelachvili JN (1977) Refinement of the fluid-mosaic model of membrane structure. Biochim Biophys Acta 469(2):221–225
Jamieson DJ (1998) Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 14(16):1511–1527
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53(379):2401–2410
Jørgensen K, Mouritsen OG (1995) Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys J 69(3):942–954
Klipp E, Nordlander B, Krüger R, Gennemark P, Hohmann S (2005) Integrative model of the response of yeast to osmotic shock. Nat Biotechnol 23(8):975–982
Klis FM, Mol P, Hellingwerf K, Brul S (2002) Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol Rev 26(3):239–256
Krallish I, Jeppsson H, Rapoport A, Hahn-Hägerdal B (1997) Effect of xylitol and trehalose on dry resistance of yeasts. Appl Microbiol Biotechnol 47(4):447–451
Kuge S, Jones N (1994) YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J 13(3):655
Laroche C, Beney L, Marechal P, Gervais P (2001) The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl Microbiol Biotechnol 56(1–2):249–254
Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B (2002) The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys J 82(4):2101–2110
Leckband D, Helm C, Israelachvili J (1993) Role of calcium in the adhesion and fusion of bilayers. Biochemistry (Mosc) 32(4):1127–1140
Lee S, Sielaff B, Lee J, Tsai FT (2010) CryoEM structure of Hsp104 and its mechanistic implication for protein disaggregation. Proc Natl Acad Sci U S A 107(18):8135–8140
Lemetais G, Dupont S, Beney L, Gervais P (2012) Air-drying kinetics affect yeast membrane organization and survival. Appl Microbiol Biotechnol 96(2):471–480
Leprince O, Atherton NM, Deltour R, Hendry GA (1994) The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea mays L. (an electron paramagnetic resonance study). Plant Physiol 104(4):1333–1339
Leprince O, Harren FJ, Buitink J, Alberda M, Hoekstra FA (2000) Metabolic dysfunction and unabated respiration precede the loss of membrane integrity during dehydration of germinating radicles. Plant Physiol 122(2):597–608
Leslie SB, Teter SA, Crowe LM, Crowe JH (1994) Trehalose lowers membrane phase transitions in dry yeast cells. Biochim Biophys Acta 1192(1):7–13
Lipke PN, Ovalle R (1998) Cell wall architecture in yeast: new structure and new challenges. J Bacteriol 180(15):3735–3740
Longo VD, Gralla EB, Valentine JS (1996) Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae mitochondrial production of toxic oxygen species in vivo. J Biol Chem 271(21):12275–12280
López-Martínez G, Pietrafesa R, Romano P, Cordero-Otero R, Capece A (2013) Genetic improvement of Saccharomyces cerevisiae wine strains for enhancing cell viability after desiccation stress. Yeast 30(8):319–330
López-Martínez G, Rodríguez-Porrata B, Margalef-Català M, Cordero-Otero R (2012) The STF2p hydrophilin from Saccharomyces cerevisiae is required for dehydration stress tolerance. PLoS One 7(3):e33324
Lu J, Holmgren A (2014) The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87
Mager WH, Siderius M (2002) Novel insights into the osmotic stress response of yeast. FEMS Yeast Res 2(3):251–257
Marechal PA, de Maranon IM, Molin P, Gervais P (1995) Yeast cell responses to water potential variations. Int J Food Microbiol 28(2):277–287
Matsumoto R, Rakwal R, Agrawal GK, Jung Y-H, Jwa N-S, Yonekura M, Iwahashi H, Akama K (2006) Search for novel stress-responsive protein components using a yeast mutant lacking two cytosolic Hsp70 genes, SSA1 and SSA2. Mol Cells 21(3):381–388
Miermont A, Waharte F, Hu S, McClean MN, Bottani S, Leon S, Hersen P (2013) Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc Natl Acad Sci U S A 110(14):5725–5730
Morris G, Winters L, Coulson G, Clarke K (1986) Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae. J Gen Microbiol 132(7):2023–2034
Novichkova A, Rapoport A (1983) Intracellular pool of free amino acids in dehydrated yeast organisms. Mikrobiologiia 53(1):5–9
Ogilby PR (2010) Singlet oxygen: there is indeed something new under the sun. Chem Soc Rev 39(8):3181–3209
Orlean P (2012) Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192(3):775–818
Paciaroni A, Cinelli S, Cornicchi E, Francesco AD, Onori G (2005) Fast fluctuations in protein powders: the role of hydration. Chem Phys Lett 410(4):400–403
Persson E, Halle B (2008) Cell water dynamics on multiple time scales. Proc Natl Acad Sci U S A 105(17):6266–6271
Piette J (1991) New trends in photobiology: biological consequences associated with DNA oxidation mediated by singlet oxygen. J Photochem Photobiol B 11(3):241–260
Posas F, Chambers JR, Heyman JA, Hoeffler JP, de Nadal E, Jn A (2000) The transcriptional response of yeast to saline stress. J Biol Chem 275(23):17249–17255
Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF (1993) Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J 65(2):661–671
Rapoport A, Khroustalyova G, Crowe L, Crowe J (2009) Anhydrobiosis in yeast: stabilization by exogenous lactose. Microbiology 78(5):624–629
Rapoport A, Khrustaleva G, Chamanis G, Beker M (1995) Yeast anhydrobiosis: permeability of the plasma membrane. Microbiology 64(2):229–232
Rapoport A, Puzyrevskaya O, Saubenova M (1988) Polyols and resistance of yeasts to dehydration. Microbiology 57(2):269–271
Ratnakumar S, Hesketh A, Gkargkas K, Wilson M, Rash BM, Hayes A, Tunnacliffe A, Oliver SG (2011) Phenomic and transcriptomic analyses reveal that autophagy plays a major role in desiccation tolerance in Saccharomyces cerevisiae. Mol BioSyst 7(1):139–149
Ratnakumar S, Tunnacliffe A (2006) Intracellular trehalose is neither necessary nor sufficient for desiccation tolerance in yeast. FEMS Yeast Res 6(6):902–913
Rep M, Krantz M, Thevelein JM, Hohmann S (2000) The transcriptional response of Saccharomyces cerevisiae to osmotic shock Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem 275(12):8290–8300
Rep M, Reiser V, Gartner U, Thevelein JM, Hohmann S, Ammerer G, Ruis H (1999) Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol Cell Biol 19(8):5474–5485
Reyes JL, Rodrigo MJ, Colmenero Flores JM, Gil JV, Garay Arroyo A, Campos F, Salamini F, Bartels D, Covarrubias AA (2005) Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant Cell Environ 28(6):709–718
Rodriguez-Porrata B, Carmona-Gutierrez D, Reisenbichler A, Bauer M, Lopez G, Escote X, Mas A, Madeo F, Cordero-Otero R (2012) Sip18 hydrophilin prevents yeast cell death during desiccation stress. J Appl Microbiol 112(3):512–525
Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B (2005) Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 24(8):1537–1545
Rozenfelde L, Rapoport A (2014) Anhydrobiosis in yeast: is it possible to reach anhydrobiosis for yeast grown in conditions with severe oxygen limitation? Antonie Van Leeuwenhoek 106(2):211–217
Sales K, Brandt W, Rumbak E, Lindsey G (2000) The LEA-like protein HSP 12 in Saccharomyces cerevisiae has a plasma membrane location and protects membranes against desiccation and ethanol-induced stress. Biochim Biophys Acta 1463(2):267–278
Sano F, Asakawa N, Inoue Y, Sakurai M (1999) A dual role for intracellular trehalose in the resistance of yeast cells to water stress. Cryobiology 39(1):80–87
Schaber J, Adrover MÀ, Eriksson E, Pelet S, Petelenz-Kurdziel E, Klein D, Posas F, Goksör M, Peter M, Hohmann S (2010) Biophysical properties of Saccharomyces cerevisiae and their relationship with HOG pathway activation. Eur Biophys J 39(11):1547–1556
Schneiter R, Brügger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G (1999) Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol 146(4):741–754
Shea J-E, Brooks CL (2001) From folding theories to folding proteins: a review and assessment of simulation studies of protein folding and unfolding. Annu Rev Phys Chem 52(1):499–535
Shea J-E, Onuchic JN, Brooks CL (2002) Probing the folding free energy landscape of the src-SH3 protein domain. Proc Natl Acad Sci U S A 99(25):16064–16068
Shechter E, Rossignol B (2000) Biochimie et biophysique des membranes: aspects structuraux et fonctionnels. Dunod
Simonin H, Beney L, Gervais P (2007) Sequence of occurring damages in yeast plasma membrane during dehydration and rehydration: mechanisms of cell death. Biochim Biophys Acta 1768(6):1600–1610
Singh J, Kumar D, Ramakrishnan N, Singhal V, Jervis J, Garst JF, Slaughter SM, DeSantis AM, Potts M, Helm RF (2005) Transcriptional response of Saccharomyces cerevisiae to desiccation and rehydration. Appl Environ Microbiol 71(12):8752–8763
Slaninova I, Sestak S, Svoboda A, Farkas V (2000) Cell wall and cytoskeleton reorganization as the response to hyperosmotic shock in Saccharomyces cerevisiae. Arch Microbiol 173(4):245–252
Sleigh S, Seavers P, Wilkinson A, Ladbury J, Tame J (1999) Crystallographic and calorimetric analysis of peptide binding to OppA protein. J Mol Biol 291(2):393–415
Stadtman E, Levine R (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25(3–4):207–218
Steels E, Learmonth R, Watson K (1994) Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 140(3):569–576
Sun WQ, Leopold AC (1997) Cytoplasmic vitrification and survival of anhydrobiotic organisms. Comp Biochem Phys A 117(3):327–333
Suutari M, Liukkonen K, Laakso S (1990) Temperature adaptation in yeasts: the role of fatty acids. J Gen Microbiol 136(8):1469–1474
Tamas MJ, Luyten K, Sutherland FC, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J, Gustafsson L, Thevelein JM, Hohmann S (1999) Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol 31(4):1087–1104
Tamas MJ, Rep M, Thevelein JM, Hohmann S (2000) Stimulation of the yeast high osmolarity glycerol (HOG) pathway: evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett 472(1):159–165
Tanford C (1997) How protein chemists learned about the hydrophobic factor. Protein Sci 6(6):1358–1366
Tokumasu F, Jin AJ, Feigenson GW, Dvorak JA (2003) Nanoscopic lipid domain dynamics revealed by atomic force microscopy. Biophys J 84(4):2609–2618
Tomlin GC, Hamilton GE, Gardner DC, Walmsley RM, Stateva LI, Oliver SG (2000) Suppression of sorbitol dependence in a strain bearing a mutation in the SRB1/PSA1/VIG9 gene encoding GDP-mannose pyrophosphorylase by PDE2 overexpression suggests a role for the Ras/cAMP signal-transduction pathway in the control of yeast cell-wall biogenesis. Microbiology 146(Pt 9):2133–2146
Tunnacliffe A, Wise MJ (2007) The continuing conundrum of the LEA proteins. Naturwissenschaften 94(10):791–812
Veatch SL, Keller SL (2003) Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J 85(5):3074–3083
Veatch SL, Keller SL (2005) Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta 1746(3):172–185
Ventina EJ, Saulite LA, Rapoport AI, Beker ME (1984) Electron-microscopic study of yeasts in a state of anabiosis and reactivated from this state. Microbiology 53(4):536–541
Welch AZ, Gibney PA, Botstein D, Koshland DE (2013) TOR and RAS pathways regulate desiccation tolerance in Saccharomyces cerevisiae. Mol Biol Cell 24(2):115–128
Wiseman H (1993) Vitamin D is a membrane antioxidant ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett 326(1):285–288
Wolkers WF, van Kilsdonk MG, Hoekstra FA (1998) Dehydration-induced conformational changes of poly-L-lysine as influenced by drying rate and carbohydrates. Biochim Biophys Acta 1425(1):127–136
Wright JC (2001) Cryptobiosis 300 years on from van Leuwenhoek: what have we learned about tardigrades? Zool Anz 240(3):563–582
Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143(1):291–299
Zhang Y, Nijbroek G, Sullivan ML, McCracken AA, Watkins SC, Michaelis S, Brodsky JL (2001) Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell 12(5):1303–1314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dupont, S., Rapoport, A., Gervais, P. et al. Survival kit of Saccharomyces cerevisiae for anhydrobiosis. Appl Microbiol Biotechnol 98, 8821–8834 (2014). https://doi.org/10.1007/s00253-014-6028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6028-5